Abstract
BACKGROUND: ARDS is an important cause of respiratory failure and continues to be associated with a high mortality rate. Numerous therapeutic interventions have been employed to improve patient outcomes, including inhaled epoprostenol.
METHODS: We examined subjects with ARDS treated with epoprostenol. We compared hospital survivors with nonsurvivors to identify predictors of mortality.
RESULTS: Among the cohort (n = 216), there were 80 (37%) hospital survivors and 136 (63%) hospital nonsurvivors. Logistic regression revealed 5 variables associated with hospital mortality: trauma as the etiology for ARDS (adjusted odds ratio [AOR] 0.09, 95% CI 0.04–0.22, P = .006), presence of both pulmonary and nonpulmonary sources of sepsis (AOR 3.06, 95% CI 1.98–4.74, P = .01), an international normalized ratio of > 1.5 (AOR 3.15, 95% CI 2.19–4.54, P = .002), body mass index (1-unit increments, AOR 0.95, 95% CI 0.936–0.965, P = .001), and an incremental change in PaO2/FIO2 during the first 24 h of treatment with epoprostenol (AOR 0.99, 95% CI 0.988–0.994, P = .002). An analysis for 90-d mortality identified the same predictors, with the addition of cumulative fluid balance during treatment with epoprostenol of > 4 L also being an independent predictor (AOR 2.36, 95% CI 1.66–3.37, P = .02).
CONCLUSIONS: Although the use of epoprostenol in ARDS remains a therapeutic challenge, we were able to identify predictors of mortality for this important cohort of patients. These predictor variables could be employed in the design of future trials of epoprostenol in ARDS.
Introduction
Significant investigation and health-care dollars have been directed toward improving outcomes in ARDS. Despite these efforts, morbidity and mortality remain unacceptably high, with reported hospital mortality rates of > 30%.1,2 Only the use of low tidal volumes has consistently shown mortality benefit in trials examining ARDS.3–5 The high mortality associated with ARDS has led to the evaluation and use of salvage therapies, including pulmonary vasodilators, corticosteroids, surfactants, prone positioning, neuromuscular blockers, alternative modes of mechanical ventilation, and extracorporeal membrane oxygenation (ECMO).6–10 The utilization of these salvage modalities has traditionally been based on local factors such as availability of the technique and experience of the clinicians caring for patients with ARDS. Inhaled epoprostenol is employed as a rescue therapy for ARDS, and its use is associated with improved oxygenation, reduced shunt, and decreased pulmonary artery pressures.11 We hypothesized that specific subgroups of patients with ARDS receiving rescue therapy with inhaled epoprostenol may be more likely to survive or die. Therefore, we set out to identify predictors of hospital mortality and 90-d mortality among patients with ARDS treated with inhaled epoprostenol.
QUICK LOOK
Current knowledge
ARDS is characterized by ventilation/perfusion mismatching and hypoxemia. Inhaled vasodilators, including nitric oxide and aerosolized epoprostenol, have been used to improve oxygenation, but neither has been shown to improve survival.
What this paper contributes to our knowledge
This retrospective review of subjects with ARDS treated with aerosolized epoprostenol demonstrated a 90-d mortality rate of 68%. Subjects with trauma, greater body mass index, and greater improvements in oxygenation had an improved hospital and 90-d survival. Subjects with sepsis and an international normalized ratio of > 1.5 had an increased mortality.
Methods
We conducted a retrospective review of all patients admitted with ARDS to a large, urban, tertiary-care teaching hospital (Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri). We focused on the time period from January 1, 2004, to June 30, 2012. The study was approved by the Washington University School of Medicine Human Studies Committee and informed consent was waived (Protocol No. 201212017).
Subjects
To be included in the study population, subjects had to meet the Berlin criteria for ARDS, including the criteria for PaO2/FIO2 and PEEP.12 More specifically, the Berlin Definition for ARDS requires the presence of (1) onset within 1 week of a known clinical insult or new or worsening respiratory symptoms; (2) bilateral opacities not fully explained by effusions, lobar/lung collapse, or nodules; (3) respiratory failure not fully explained by cardiac failure or fluid overload; and (4) oxygenation impairment (minimum impairment of 200 mm Hg < PaO2/FIO2 ≤ 300 mm Hg with PEEP ≥ 5 cm H2O). Moreover, subjects had to receive inhaled epoprostenol for the treatment of ARDS to be eligible for study inclusion. One investigator (JP) reviewed all the cases and imaging studies to confirm that subjects met these inclusion criteria.
Ventilation Modes
The primary mode of ventilation for ARDS at Barnes-Jewish Hospital during this study period was volume control continuous mandatory ventilation using a tidal volume of 6–8 mL/kg based on predicted body weight (PB840 ventilator, Covidien, Mansfield, Massachusetts). Airway pressure release ventilation via the bi-level mode on the PB840 ventilator was also used during the study period at the request of the treating physicians.
Inhaled Epoprostenol Administration
Inhaled epoprostenol was provided to subjects with ARDS after a physician entered the order via a standardized electronic ordering system for inhaled epoprostenol (Flolan, epoprostenol sodium, GlaxoSmithKline, Philadelphia, Pennsylvania), starting at a dose of 20,000 ng/mL. Respiratory care practitioners administered the inhaled epoprostenol utilizing an infusion pump and a low-flow jet nebulizer (Mini-HEART, Medline Industries, Mundelein, Illinois) that was in line with the ventilator circuit. The syringes employed for inhaled epoprostenol were carefully labeled to indicate that they were to be used only via the inhalational route. The dose of epoprostenol was weaned as the subject responded to the administered therapy. The Mini-HEART nebulizer was charged with a volume of 15 mL of epoprostenol. The infusion rate for epoprostenol was 8 mL/h into the Mini-HEART nebulizer. The respiratory therapist connected the oxygen tubing from the nebulizer to an oxygen flow meter and set the flow at 2–3 L/min. At this flow, the nebulizer output was ∼8 mL/h. The flow of gas to the nebulizer may need adjustment to maintain 15 mL of the epoprostenol solution in the reservoir at all times. Increasing or decreasing the gas flow changed the nebulizer output. Nebulizer flow must not exceed 3 L/min.
End Points
The primary end point was hospital mortality. We also examined 90-d mortality as a secondary end point.
Definitions and Covariates
Fluid balance was determined by assessing the electronic medical records for 2 time frames: during hospitalization before the start of epoprostenol and during the infusion period for epoprostenol. Appropriate empiric antimicrobial therapy was defined as antimicrobials given within 24 h of the onset of signs and symptoms of infection that were active against the pathogen(s) associated with infection based on in vitro susceptibility testing.13 Severity of illness was assessed by Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment scores.14,15 Pulmonary sources of sepsis included pneumonia, lung abscess, and empyema. Nonpulmonary sources of sepsis included bacteremia (not related to a pulmonary source of infection), intra-abdominal infection, skin or wound infection, and urinary tract infections. In addition, we recorded information regarding subject demographics (ie, age, gender, race) and processes of care (use of corticosteroids, neuromuscular blocking agents, administration of nitric oxide).
Statistics
We compared hospital survivors with nonsurvivors. To compare categorical variables, we utilized the chi-square test and the Fisher exact test. For continuous variables, we employed either the Student t test or nonparametric tests, as appropriate. All tests were 2-tailed, and we assumed statistical significance if P < .05.
To identify factors independently associated with hospital mortality, we relied on logistic regression. Variables significant at the .15 level that were considered biologically relevant were entered into the regression model. We assessed variables for co-linearity and explored goodness of fit based on the Hosmer-Lemeshow test. Potential interactions were examined with the Breslow-Day test. Analyses were performed using SPSS 11.0 (SPSS, Chicago, Illinois).
Results
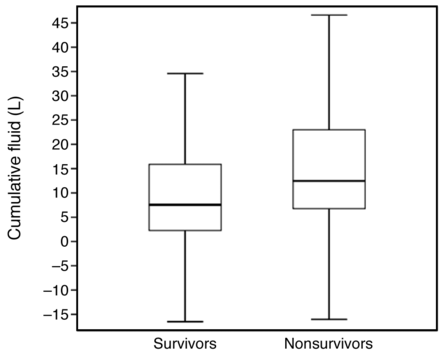
The entire cohort included 216 subjects. There were 80 (37%) hospital survivors and 136 (63%) hospital nonsurvivors. Table 1 shows the clinical characteristics according to hospital survival. Process-of-care variables are summarized in Table 2. The mean and median epoprostenol doses administered during the first 24 h of therapy and at the end of therapy were significantly greater for hospital nonsurvivors, although the duration of epoprostenol administration was shorter for hospital nonsurvivors. Epoprostenol-free days during the first 72 h of mechanical ventilation were statistically greater among survivors, although the absolute difference was small (< 1 d). Stress-dose administration of corticosteroids was statistically greater among nonsurvivors, as was the use of norepinephrine and epinephrine as vasoactive agents. Cumulative fluid balance was greater during the period of hospitalization before epoprostenol administration and during epoprostenol treatment, only achieving statistical significance during the treatment phase. Figure 1 shows the total cumulative fluid balance for survivors and nonsurvivors up to the end of epoprostenol treatment (median [interquartile range] of 7.6 L [2.3–15.9 L] for survivors and 12.5 L [6.7–23.2 L] for nonsurvivors, P = .001). PaO2/FIO2 was significantly greater in survivors compared with nonsurvivors during the initiation, first 24 h, and termination of epoprostenol treatment (Fig. 2). ICU stay was significantly longer for survivors compared with nonsurvivors (23.6 ± 14.0 vs 14.2 ± 13.9 d, P < .001).
Subjects Characteristics
Process-of-Care Variables
Box plots depicting cumulative fluid balance up to the termination of epoprostenol for hospital survivors and nonsurvivors. The lines within the boxes represent the 50th percentile, the lines at the bottom and top of the boxes represent the 25th and 75th percentiles, and the whisker lines represent the 5th and 95th percentiles. P = .001.
Mean values ± 95% confidence intervals for PaO2/FIO2 in hospital survivors (solid bars) and nonsurvivors (hatched bars) at three distinct times.
Logistic regression (Table 3) identified 5 variables independently associated with hospital mortality. Lower risk of hospital mortality was associated with trauma as the etiology for ARDS, increasing body mass index (1-unit increments), and an incremental change in PaO2/FIO2 during the first 24 h of treatment with epoprostenol, whereas greater risk of hospital mortality was associated with presence of both pulmonary and nonpulmonary sources of sepsis and an international normalized ratio of > 1.5.
Independent Factors Associated With Hospital Mortality
There were 68 (31.5%) 90-d survivors and 148 (68.5%) 90-d nonsurvivors. Logistic regression identified 6 variables independently associated with 90-d mortality. Lower risk of hospital mortality was associated with trauma as the etiology for ARDS, increasing body mass index (1-unit increments), and an incremental change in PaO2/FIO2 during the first 24 h of treatment with epoprostenol, whereas greater risk of hospital mortality was associated with presence of both pulmonary and nonpulmonary sources of sepsis, an international normalized ratio of > 1.5, and cumulative fluid balance during treatment with epoprostenol of > 4 L (Table 4).
Independent Factors Associated With 90-d Mortality
Discussion
This retrospective analysis of subjects with ARDS treated with inhaled epoprostenol confirms the high risk of mortality in this population. Although ARDS patients with a greater risk of mortality may be more likely to be subjected to treatment with salvage therapies, they may also be less likely to respond to such treatments. The identification of patients who are unlikely to survive such interventions could improve the overall utilization of rescue treatments. Moreover, the unique factors identified as predictors of outcome could be employed in the design of future clinical trials of epoprostenol for ARDS by standardizing supportive therapies such as fluid administration and ensuring that balance is achieved in important baseline characteristics such as body weight, ARDS etiology, and causes of sepsis.
Epoprostenol is the inhaled pulmonary vasodilator primarily employed at Barnes-Jewish Hospital for patients with ARDS, chiefly due to the lower cost associated with its use. Inhaled nitric oxide is less commonly employed but has more clinical trials examining its use. In randomized clinical trials, inhaled nitric oxide was associated with a transient improvement in oxygenation in adults with ARDS without any survival benefit.16–20 In a systematic review and meta-analysis of patients with acute lung injury or ARDS from 12 trials, inhaled nitric oxide was associated with modest improvements in oxygenation (13% increase in PaO2/FIO2 until days 3–4 of administration), no effect on mean pulmonary artery pressure, and no effect on survival or duration of mechanical ventilation.21 Available clinical data suggest that epoprostenol and inhaled nitric oxide are associated with similar improvements in oxygenation and outcomes in patients with ARDS.6,22,23 However, available clinical trial data do not resolve the question of whether pulmonary vasodilators lead to any clinically important benefits in certain subgroups of patients, such as those with severe hypoxemia not responding to conventional treatment. It is in this sense that pulmonary vasodilators are frequently used as a rescue therapy. Our experience with epoprostenol in ARDS suggests that groups of patients having either greater or lesser likelihood of survival can be identified.
Other authors have attempted to develop criteria aimed at identifying patients with ARDS who are more likely to have good outcomes associated with salvage therapies. Camporota et al24 examined subjects with ARDS receiving high-frequency oscillatory ventilation. Improvement in the PaO2/FIO2 of > 38% occurring at any time within the first 72 h was the best predictor of survival at 30 d. Multivariate analysis showed that high-frequency oscillatory ventilation was more effective in younger subjects when instituted early and in subjects with milder respiratory acidosis.24 Pappalardo et al25 developed a score to predict survivors of ARDS associated with influenza A (H1N1) treated with ECMO. The predictor variables in their score included hospital stay before ECMO institution, bilirubin, creatinine, hematocrit, and mean arterial pressure. In a similar analysis of ARDS attributed to H1N1, Pham et al26 identified older age, higher lactate, and higher plateau pressures during ECMO as being associated with increased odds of ICU death for ECMO recipients and demonstrated no overall survival benefit with the use of ECMO. However, these types of outcome data provide an opportunity to design therapeutic intervention trials in ARDS targeting patient populations that may be more likely to demonstrate benefit.
Our study is similar to that of Pham et al26 in identifying a process-of-care variable that was associated with excess mortality in ARDS subjects receiving a specific rescue therapy, namely greater fluid balance during the administration of epoprostenol compared with greater achieved plateau pressures in their study. Other investigators have also found associations between fluid balance and outcome in subjects with ARDS27,28 and those at risk for this syndrome.29,30 This has potentially important implications in terms of optimizing patient outcomes when inhaled vasodilator therapy or other rescue therapies are employed, especially in the context of a clinical trial. Our study is unique in identifying greater body mass index as a predictor of survival. Previous studies have shown that obesity is associated with the development of ARDS.31,32 However, a recent French study found the use of prone positioning to be safe in obese subjects, and prone positioning improved oxygenation more in obese subjects with ARDS than in non-obese subjects.33
Our study has several important limitations. First, it is retrospective and therefore prone to several forms of bias. We attempted to minimize the impact of this by confirming, through the use of multiple hospital databases, that all patients with ARDS who received therapy with inhaled epoprostenol were included in this analysis. Moreover, we had one investigator determine that each subject met the criteria for ARDS, including the radiographic criteria. Second, we did not track the use of airway pressure release ventilation within this study, nor did we track the use of prone positioning. Therefore, we could not determine whether the use of these salvage therapies influences the outcomes associated with epoprostenol use. Third, the data came from a single center, limiting its more general applicability. Fourth, in restricting the analysis to patients receiving inhaled epoprostenol as a rescue therapy, we may have skewed our findings by focusing on patients with more severe disease. This is supported by the high mortality (> 60%) and relatively low PaO2/FIO2 we observed. This limits the applicability of these data to patients with less severe disease. Finally, our sample size was somewhat limited. With a greater number of subjects, we could have employed differing approaches to modeling the risk for hospital and 90-d mortality and better validated the identified predictor variables.
Unfortunately, there are few randomized data regarding the use of inhaled epoprostenol for ARDS.34 Although we know that inhaled epoprostenol can improve oxygenation and reduce shunt11 in patients with ARDS, there are no compelling data that its routine use will improve survival.6,22,23,34 Moreover, there are virtually no data to guide clinicians in terms of which patients with ARDS are more likely to benefit from the administration of inhaled epoprostenol. Nevertheless, our findings suggest that the use of inhaled epoprostenol in patients with ARDS associated with trauma, greater body mass index, and more robust improvements in oxygenation is more likely to result in both hospital and 90-d survival.
In conclusion, patients receiving rescue therapies such as inhaled epoprostenol typically have a high risk for hospital and 90-d mortality. Although few potential rescue therapies for ARDS, including prone positioning and administration of neuromuscular blockers,8,33,35 have been shown to improve survival, it is likely that the use of rescue therapies will continue and even expand due to the high mortality associated with this syndrome and the lack of more definitive treatments. The identification of outcome predictors associated with the use of inhaled epoprostenol for ARDS may facilitate more accurate identification of patients who are unlikely to benefit from its administration. This could result in more focused and cost-effective use of epoprostenol and improve future clinical trial design by allowing more balanced groups for comparison and improved inclusion and exclusion criteria to enhance detection of a therapeutic effect.
Footnotes
- Correspondence: Marin H Kollef MD, Division of Pulmonary and Critical Care Medicine, Washington University School of Medicine, 660 South Euclid Avenue, Campus Box 8052, St. Louis, MO 63110. E-mail: mkollef{at}dom.wustl.edu.
Dr Kollef was supported by the Barnes-Jewish Hospital Foundation. The other authors have disclosed no conflicts of interest.
See the Related Editorial on Page 1312
- Copyright © 2014 by Daedalus Enterprises