Abstract
Pacemaker endocarditis has a high rate of morbidity and mortality and is associated with substantial health-care cost. To maximize the effectiveness of treatment, diagnosis of pacemaker endocarditis should be made as early as possible. Medical treatment alone is not successful, and the removal of the entire artificial pacing system is often required. We present a case of a female patient with a permanent transvenous pacemaker, recurring episodes of fever and chills, general malaise, and a computed tomography image of a solitary tumor-like lesion indicating pneumonia. The symptoms subsided with empirical antibiotics but without improvement in the radiologic images. A wedge resection of the lesion by thoracotomy was performed, revealing a necrotic lung lesion compatible with pulmonary infarct. Transesophageal echocardiography showed a mass that was adherent to the pacemaker lead. The therapeutic approach consisted of surgical removal of the complete pacing system along with long-term antibiotic therapy and implantation of a new device with an epicardial lead. Serial follow-up echocardiograms for a 1-y period did not show any recurrence, and the subsequent course was uneventful.
- infective endocarditis
- pacemaker infection
- echocardiography
- pulmonary infarct
- septic pulmonary emboli
- management of cardiac device-related infective endocarditis
Introduction
The risk of pacemaker-related infective endocarditis has increased since the evidenced-based indications of these devices have progressively expanded.1 Permanent pacemaker infection is a serious complication, with reported rates varying between 0.13% and 19.9%.2 Pacemaker endocarditis accounts for 10% of pacemaker-associated infections and has a mortality rate of 30–35%.2–5 Extended and serious forms of this infection may affect the leads, the cardiac valves, or the endocardiac surface and are called cardiac device-related infective endocarditis (CDRIE). Its clinical presentation may vary, making diagnosis difficult. We report a case of CDRIE for which diagnosis was delayed because of its atypical presentation. A solitary pulmonary lesion revealed by chest computed tomography (CT) was initially misdiagnosed as round pneumonia.
Case Report
We report a case of a 55-y-old female thalassemia carrier with a medical history of arterial hypertension, type 2 diabetes mellitus, and a known allergy to cephalosporins. The subject received an implanted permanent transvenous pacemaker (St Jude Medical, Saint Paul, Minnesota; DDDR (Dual chamber paces, Dual chambers sensed, Dual response, Rate modifiable) mode, rate of 60 beats/min) due to sick sinus syndrome. Three years later, she developed a high-grade fever (39°C) and chills. Associated symptoms included general malaise and musculoskeletal pain in the lower limbs. Laboratory tests revealed an elevated white blood cell count, and the plain chest x-ray demonstrated right lower lobe infiltrate, which was diagnosed as pneumonia. She had been treated with empirical antibiotic therapy for community-acquired pneumonia as an out-patient by the family doctor. Although her symptoms improved, she still suffered from musculoskeletal pain. Three months later, she was referred to our hospital (General Hospital of Thessaloniki G. Papanikolaou, Thessaloniki, Greece) because of a persistent high fever and right lower lobe infiltrate. On admission, physical examination revealed no pathological findings. Chest CT scan revealed a solitary tumor-like consolidation located in the posterior basal segment of the right lower lobe (5.5 × 4.5 × 3 cm) abutting the pleura (Fig. 1). This was interpreted as round pneumonia. The 2 pacemaker leads were in normal positions. An electrocardiogram showed a good functioning pacemaker. The subject received moxifloxacin for 1 week. She became afebrile with a normal white blood cell count, but the other markers of inflammation, such as erythrocyte sedimentation rate and C-reactive protein, were still elevated. Further investigations, including multiple blood and sputum cultures, immunology tests, and screening for vasculitis, were all negative. The subject underwent bronchoscopy with normal findings. Microbiological and cytological analyses of bronchoalveolar lavage, along with a protected bronchial brushing, were negative as well. However, the diagnosis of infection related to the pacemaker had not been considered in the differential diagnosis.
Computed tomography scan images of the lung showing a solitary pleural-based lesion in the right lower lobe.
Upon a follow-up examination, the subject showed no radiological improvement. Two months later, the tumor-like solitary pulmonary nodule was still present. She underwent right thoracotomy, and a wedge resection of the lesion was performed. Macroscopic evaluation of the resected tissue revealed a necrotic lesion. Microscopic examination showed coagulation-type necrotic tissue, recanalization, a large amount of eosinophilic material, and degradation of nuclei. The necrotic area was surrounded by fibrotic tissue. Inside the necrotic lesion, ghost vessels and vessels with intraluminal obliteration and hemosiderin-containing alveolar macrophages were observed. These findings were compatible with pulmonary infarct.
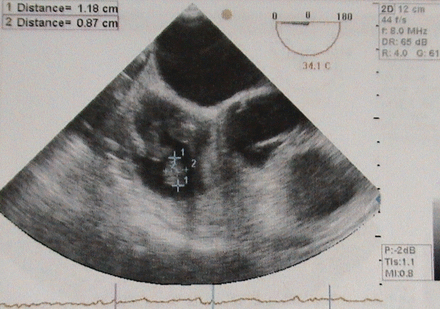
On postoperative d 10, she became febrile again (maximum of 40°C), with an elevated white blood cell count. She complained of malaise, anorexia, and weight loss. Although there were no local symptoms of inflammation at the site of pacemaker implantation, pacemaker endocarditis was suspected due to ongoing fevers and the histopathologic findings. As a result, the subject underwent a transthoracic echocardiography exam, but this did not yield a diagnosis. Further investigation by transesophageal echocardiography revealed a large oscillating intracardiac mass in the right atrium (11.8 × 8.7 mm) attached to the pacemaker lead (Fig. 2). Systolic function was normal, and there was moderate tricuspid regurgitation. Blood cultures were again negative. Therefore, empirical intravenous antistaphylococcal therapy for endocarditis was initiated (vancomycin at 15 mg/kg/12 h and gentamycin at 1 mg/kg/8 h) because staphylococci are involved in the majority of these infections.
Transesophageal echocardiogram: view of the right atrium. There is a round vegetation (1.18 × 0.87 cm) attached to the pacemaker lead.
Surgical removal of the entire pacing system using extracorporeal support of circulation was the decided course of action. The subject underwent median sternotomy. The pacemaker device and leads were extracted under extracorporeal circulation (cardiopulmonary bypass time 30 min) and were replaced with an epicardial atrioventricular pacemaker because the subject was pacemaker-dependent. Intraoperative transesophageal echocardiography showed complete removal of the vegetation, leaving the valves and endocardiac surfaces intact. Cultures from the extracted pacemaker leads and vegetation were negative. Antimicrobial therapy with vancomycin was continued for a total of 6 weeks.
The postoperative period was uneventful. The subject remained afebrile with a normal white blood cell count. There was no recurrence 1 y after she was discharged from the cardiothoracic department.
Discussion
As noted, reported rates of permanent pacemaker infection may reach 19.9%.2 Pacemaker endocarditis accounts for 10% of pacemaker-associated infections and is a potentially lethal complication.2–5 Factors contributing to a higher incidence of infection include age, diabetes mellitus, immunosuppression, neoplasm, anticoagulant use, intravenous catheters, temporary pacing, early re-intervention after pacemaker implantation, dermatological diseases, absence of periprocedural antibiotic prophylaxis, and other infectious foci before implantation.
Presentation of pacemaker-associated infection includes acute endocarditis caused by virulent pathogens that may lead to severe sepsis and subacute or chronic infection with nonspecific symptoms. This may obscure the initial assessment. Infection during the first year after implantation is characterized as surgical site infection. Beyond the first year, these infections are termed late-onset lead endocarditis. Permanent pacemaker infection can be limited to the pocket of the cardiac device, characterized by local signs of inflammation, or it may cause a more extended and serious infection affecting the leads, the cardiac valves, or the endocardiac surface, also called CDRIE. Early infections are most commonly caused by Staphylococcus aureus, whereas late infections are most commonly caused by coagulase-negative staphylococci such as Staphylococcus epidermidis. Other pathogens, such as Gram-positive cocci, Gram-negative bacilli, and fungi (Candida), have also been described.2,4 Positive cultures, from the blood or the subcutaneous pacemaker pocket, have high diagnostic value and lead to appropriate treatment. Blood cultures are positive in 77% of patients suffering from CDRIE. Prior antibiotic administration or organisms with limited proliferation under conventional culture conditions may underlie many of the observed instances of culture-negative endocarditis, often delaying the diagnosis and onset of treatment. Antibiotic selection should be based on the identified pathogen and guided by antimicrobial susceptibility testing. Prolonged, parenterally-administered bactericidal therapy should be administered for complicated infections. In the case of negative cultures when pacemaker-related infective endocarditis is suspected, broad-spectrum antibiotic therapy must be instituted.2–4
Transthoracic echocardiography is an important diagnostic tool in detecting endocarditis, particularly when blood cultures are negative. Echocardiography is indicated for the measurement of vegetation size and is also useful for detecting complications, such as valvular insufficiency, congestive heart failure, and paravalvular abscesses, and for predicting embolic events. When the transthoracic echocardiogram is negative or inconclusive and there is a high clinical suspicion of infective endocarditis, transesophageal echocardiography should be performed. In cases in which pacing wire endocarditis is suspected, it is difficult to assess by transthoracic echocardiography whether tricuspid valve endocarditis, pacemaker lead infection, or both are present. Transesophageal echocardiography has better diagnostic accuracy compared with transthoracic echocardiography for this particular application, with high sensitivity (94% vs 23%, respectively).6 There is a 5% possibility that the identified intracardiac mass that is adhering to the lead is a thrombus and not infected vegetation, and there is no need for lead removal or antibiotic treatment in these cases. We conclude that, in addition to positive echocardiographic findings, clinical features and laboratory data are essential to establish the diagnosis of endocarditis. In cases in which surgical removal of the pacing system is deemed necessary, intraoperative transesophageal echocardiography is recommended because it may provide additional diagnostic information and modify the surgical plan. Echocardiography should also be repeated after device extraction.7
CDRIE is a heterogeneous disease with variable clinical presentations. The use of modified Duke criteria is suggested for correct diagnosis of the condition. Our subject fulfilled these criteria for definite diagnosis of infective endocarditis: 1 major, echocardiogram evidence of infective endocarditis; and 3 minor, predisposition, fever, and pulmonary infarct. Although these criteria are of considerable use, they must not replace clinical judgment. CDRIE must be suspected in the presence of unexplained persisting fever in all patients with an implanted pacemaker even in the absence of local signs of infection or negative blood cultures. In our case, cultures from the extracted pacemaker leads and vegetation were also negative. However, it has been reported that only 25.4% of cultured valves from patients with infected endocarditis could be true positive.8
In the case presented here, pacemaker lead infection was not initially considered in the differential diagnosis. The negative blood cultures, the absence of local signs of infection at the generator pocket site, the long time between pacemaker implantation and occurrence of infection, and the presence of specific findings in the chest CT scan suggesting pneumonia significantly delayed the correct diagnosis.
Infective endocarditis can cause pulmonary complications, especially septic emboli. Emboli to the lungs often present with infective endocarditis. Patients with large vegetations (> 10 mm) are at high risk of embolism, whereas the risk of a new embolism is highest during the first days following the initiation of antibiotic therapy. Imaging, together with clinical signs and laboratory tests, plays a major role in the diagnosis of septic pulmonary emboli. Although radiographic abnormalities may be nonspecific, typical lung CT findings include bilateral, poorly defined, parenchymal nodules or multifocal infiltrates, commonly involving peripheral lung zones, often associated with varying degrees of cavitation.5 There is also a tendency for the septic nodules to change appearance over time. Feeding vessels are observed in nodules in 60–70% of patients.9
In this case, although the subject with an implanted cardiac device was febrile and had elevated markers of systemic inflammation, the diagnosis was further delayed because of the atypical pattern of lung involvement assessed by CT. The observed pulmonary lesion was solitary without cavitary changes, and the presence of a feeding vessel was not observed.
Current recommendations for managing patients with pacemaker endocarditis include the removal of the entire pacemaker system, followed by long-term appropriate antibiotic therapy targeting the isolated organisms.10 Conservative treatment alone is rarely successful and is associated with a high risk of recurrence. The re-infection rate has been reported to be 0.8% when the device is removed and 50% if it is not removed. When antibiotic administration is the only therapeutic intervention, reported mortality rates range from 31 to 66%, whereas the mortality rate is 18% when extraction of the device is accompanied by medical treatment.2,4 There are 2 surgical options for removing the pacemaker leads: percutaneous or open surgical techniques. The decision as to which technique to use should be based on vegetation size, duration of implantation, age of the patient, and coexisting conditions.11
Percutaneous lead extraction is the preferred method for removal of CDRIE hardware, although this method carries risks of arrhythmias, tricuspid valve tear, or right ventricle damage, especially in patients who have pacemakers for prolonged periods. Over time, the intravascular pacemaker leads can be encapsulated deeply into right ventricular tissue, making extraction difficult. There are many techniques for percutaneous lead extraction. The simplest method is direct gentle manual traction. An intravascular approach through the transvenous removal of the endocardial leads using wire-loop snares, hook-tipped wires, grasping forceps, basket retrievers, or locking stylets is an alternative.12 The risk of embolic events in the presence of large (> 20 mm) vegetations is considered a relative contraindication to transvenous removal. Another feared complication of the transvenous lead extraction technique is cardiac tamponade (0–3.3%). According to the current literature, percutaneous lead removal is feasible and safe even when the vegetation size is up to 40 mm, but a high level of experience is required.13 Surgical extraction should be considered when percutaneous extraction is incomplete or impossible, a large vegetation is present, or there is an associated destruction of the tricuspid valve.2,4 The presence of long-term leads (> 12 mo after implantation) is also a strong indication for surgical intervention by cardiopulmonary bypass.3 Immediate re-implantation should be delayed. If a pacemaker is necessary, implantation of a pacemaker system with epicardial leads is advised. We decided on pacemaker lead removal by surgical treatment in our subject. The main reasons for this were the long time period that the pacemaker had been in place and the vegetation size (> 10 mm).
Since the number of implanted cardiac devices has significantly increased, the incidence of CDRIE has concomitantly increased. Pacemaker endocarditis is associated with substantial morbidity, mortality, and increase in hospital costs. There is a need for earlier suspicion and recognition of the clinical signs of CDRIE and the proper management of this condition. The clinical presentation may be atypical, and the presenting symptoms may appear years after pacemaker implantation. Septic pulmonary emboli may be the key presenting feature of CDRIE. Although imaging plays an important role in identifying septic pulmonary emboli, atypical lung involvement must also be considered when making a correct diagnosis.
Footnotes
- Correspondence: Charilaos-Panagiotis C Koutsogiannidis MD, Cardiothoracic Surgery Department, General Hospital of Thessaloniki G. Papanikolaou, Exohi, 57010 Thessaloniki, Greece. E-mail: harisdoc76{at}yahoo.gr.
The authors have disclosed no conflicts of interest.
- Copyright © 2014 by Daedalus Enterprises