Abstract
BACKGROUND: Gas exchange measurements for carbon dioxide elimination (V̇CO2) and oxygen consumption (V̇O2) have been used to derive resting energy expenditure and guide energy prescription. Volumetric capnography is used in intensive care units and provides V̇CO2 measurements that could be used for titrating respiratory and nutritional support. We have recently suggested that measuring V̇CO2 may be sufficient to obtain a reasonable estimate of energy expenditure. However, data describing the accuracy of gas exchange measurement devices are limited.
METHODS: We used an in vitro simulation model to test the accuracy of gas exchange measurements by 2 devices: the CCM Express indirect calorimeter and the NM3, a volumetric capnography monitor. A Huszczuk gas injection system combined with a high-fidelity lung simulator was used to simulate V̇O2 and V̇CO2 values in the pediatric and adult range. Bland-Altman analysis was used to examine the agreement between the measured and simulated values across a range of tidal volumes and gas exchange values. Additionally, agreement between the 2 devices was examined.
RESULTS: During the adult simulation with the CCM Express, the mean bias (95% CI) for V̇CO2 values was −12.6% (−16.4 to −8.8%) and −17.5% (−19.9 to −15.1%) for V̇O2 values. For the pediatric simulation with the CCM Express, mean bias for V̇O2 was −14.7% (−16.4 to −13.0%) and V̇CO2 was −10.9% (−13.5 to −8.3%). For the adult and pediatric simulations with the NM3, the bias for V̇CO2 was −8.2% (−15.7 to −0.7%) and −8.3% (−19.4 to −2.8%), respectively. Between the 2 devices, the mean bias was −4.4% (−10.2 to 1.3%) and −2.3% (−11.4 to 6.8%) for the adult and pediatric V̇CO2 simulations, respectively.
CONCLUSIONS: Currently available portable gas exchange monitors demonstrated acceptable agreement with reference V̇O2 and V̇CO2 values in an in vitro simulation. The devices demonstrated good agreement with each other.
Introduction
Accurate measurements of oxygen consumption (V̇O2) and/or carbon dioxide elimination (V̇CO2) may facilitate the prescription of optimal nutrition, titration of mechanical ventilation, and assessment of substrate oxidation in pediatric and adult subjects during critical illness.1–4 Indirect calorimeters and gas exchange monitors are now being used in out-patient clinics, in-patient settings, and even home environments.5–7 Although a number of portable gas exchange monitoring devices are now commercially available, there is a paucity of data on validation of individual devices and on agreement between such devices. Therefore, we sought to examine the accuracy of an indirect calorimeter and a volumetric capnography device using a gas exchange simulator across a range of pediatric and adult respiratory values.
QUICK LOOK
Current knowledge
Carbon dioxide elimination (V̇CO2) and oxygen consumption (V̇O2) are used to calculate resting energy expenditure and guide energy prescription. We recently proposed that measuring V̇CO2 alone may be sufficient to approximate energy expenditure measured by indirect calorimetry. However, data describing the accuracy of portable devices to measure gas exchange are limited.
What this paper contributes to our knowledge
The currently available portable gas exchange monitors tested in the present investigation were in reasonable agreement with reference V̇O2 and V̇CO2 values in an in vitro simulation. Agreement between the devices was good.
Methods
A laboratory simulation model was constructed that allowed precise control of respiratory mechanics and gas exchange parameters; the model incorporated a high-fidelity lung simulator and a mass flow controller. Gas exchange simulation was based upon gas dilution and permitted the control of different levels of V̇CO2 and V̇O2.8 We aimed to simulate gas exchange values for 2 discrete subject sizes along with an array of appropriate respiratory mechanics that correspond to values observed in an ICU.
Gas Exchange Monitors
We tested 2 portable (table top) gas exchange monitors: an indirect calorimeter, the CCM Express (MGC Diagnostics, Saint Paul, Minnesota), and a volumetric capnography monitor, the NM3 (Philips Healthcare, Eindhoven, Netherlands), which measures V̇CO2. Each device was calibrated according to the manufacturer's specifications before testing, and appropriate airway adapters were used. All volumes were corrected to testing conditions (ambient temperature and pressure).
Lung Mechanics Simulation
The ASL 5000 breathing simulator (Ingmar Medical, Pittsburg, Pennsylvania) was used to control tidal volumes, inspiratory times, breathing frequencies, and compliance. For the pediatric simulation (a 35-kg subject) the pulmonary compliance was set at 35 mL/cm H2O with breathing frequency of 25 breaths/min, inspiratory time of 0.9 s, and tidal volumes (VT) set to 175, 245, and 315 mL, corresponding to 5, 7, and 9 mL/kg, respectively. For the adult simulation (a 70-kg subject) the pulmonary compliance was set at 70 mL/cm H2O, with breathing frequency of 20 breaths/min, inspiratory time of 1.1 s, and VT set to 350, 490, and 630 mL, corresponding to 5, 7, and 9 mL/kg, respectively. The accuracy of volume delivery with the ASL 5000 is ±2%.
Gas Exchange Simulation
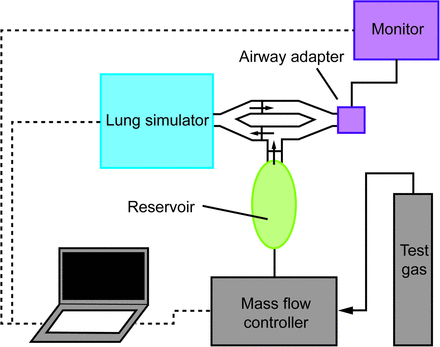
The gas injection model used to simulate V̇O2 and V̇CO2 has been described previously.8 A schematic of the experimental setup is shown in Figure 1. A mass flow controller (FMA-2605A, Omega Engineering, Stamford, Connecticut) was utilized to control the injection of a specialized test gas composed of 21% CO2, with the balance nitrogen. Accuracy of the mass flow controller is ±0.8% of the measurement reading. A customized baffle and reservoir assembly was constructed in accordance with previous methods in order to appropriately integrate the test gas into the breathing simulator. Test gas accumulates in the reservoir during exhalation. During the inspiratory cycle, one-way valves directed test gas from the reservoir, which mixes with inspired ambient air and is mixed inside the lung simulator. Upon exhalation, one-way valves direct the mixed gas away from the reservoir to prevent any more mixing and out past the airway adapter. For V̇CO2 simulation, the volume of CO2 injected can be calculated by multiplying the CO2 fraction of the test gas by the flow (0.21 × test gas flow). For V̇O2, the inspired gas is diluted by a known amount of N2 and CO2. The relationship between FIO2, inspired gas volume, and total gas volume is shown in Equation 1. Of note, this relationship can be simplified for a test gas with the CO2 content equal to the ambient FIO2 (V̇O2 = 0.21 × test gas flow). For both the pediatric and adult simulations, the system was set to mimic values at a level typically observed and also 20% above and 20% below this level.9,10 For the pediatric model, V̇O2 and V̇CO2 were simulated at 112, 140, and 168 mL/min; corresponding to 3.2, 4, and 4.8 mL/kg/min.11 For the adult model, V̇O2 and V̇CO2 were simulated at 168, 210, and 252 mL/min, corresponding to 3.2, 4, and 4.8 mL/kg/min,
 (1) where V̇i is the inspired volume per unit time of ambient air, and V̇total is the sum of V̇i and the total test gas flow.
(1) where V̇i is the inspired volume per unit time of ambient air, and V̇total is the sum of V̇i and the total test gas flow.
Schematic of the experimental setup. The small arrows represent the direction of gas flow through a one-way valve. Test gas accumulates in the reservoir during exhalation. During the inspiratory cycle, one-way valves direct test gas from the reservoir, which mixes with inspired ambient air and is mixed inside the lung simulator. Upon exhalation, one-way valves direct the mixed gas away from the reservoir to prevent any more mixing and out past the airway adapter of the gas exchange monitor.
The overall aim was to subject the devices to testing levels across the range of expected VTs, gas exchange, and other parameters typically observed in critically ill pediatric and adult subjects. Each device was tested across 9 distinct combinations of VTs and gas exchange values for the pediatric model and 9 combinations for the adult model.
Statistical Analysis
Bland-Altman analysis was used to quantify the accuracy of the devices by comparison of gas exchange measurements for each device and the set values on the simulator.12 Mean bias and 95% CIs were computed. Further, the agreement of V̇CO2 measurements between the devices was done using Bland-Altman analysis. For the purpose of this experiment, we decided a priori that limits of agreement within ±20% would be deemed clinically acceptable.13 Analyses were conducted using Prism 5.04 (GraphPad Software, La Jolla, California).
Results
A total of 18 unique combinations of gas exchange values and VT levels were tested, each of which was recorded for a 5-min period. For the adult simulation of V̇CO2, the mean biases (95% CIs) for the CCM Express and NM3 compared with the simulated reference values are shown in Table 1 and depicted in Figure 2, A and B. The mean bias (95% CI) for adult V̇CO2 readings between the 2 devices was −4.4% (−10.2 to 1.3%) (Fig. 2C).
Results From the Experiment
Bland-Altman plots of carbon dioxide elimination (V̇CO2) results in the adult range compared with the simulated values and with each other. MGC Diagnostics CCM Express indirect calorimeter (A) and Philips Healthcare NM3 (B), where difference (%) = 100 × (measured value − set value)/[(measured value + set value)/2] versus average of the measured value and set value. C: Comparison of MGC with NM3, where difference (%) = 100 × (CCM − NM3)/[(CCM + NM3)/2] versus average.
For the pediatric simulation of V̇CO2, the mean biases (95% CI) for V̇CO2 values for the devices are shown in Table 1 and depicted in Figure 3, A and B. The mean (95% CI) bias for pediatric V̇CO2 readings between the 2 devices was −2.3% (−11.4 to 6.8%) (Fig. 3C).
Bland-Altman plots of carbon dioxide elimination (V̇CO2) result in the pediatric range compared with the simulated values and with each other. MGC Diagnostics CCM Express indirect calorimeter (A) and Philips Healthcare NM3 (B), where difference (%) = 100 × (measured value − set value)/[(measured value + set value)/2] versus average of the measured value and set value. C: Comparison of MGC with NM3, where difference (%) = 100 × (CCM − NM3)/[(CCM + NM3)/2] versus average.
For the measurement of V̇O2 using the CCM Express, mean bias (95% CI) for the adult and pediatric range are shown in Table 1 and depicted in Figure 4, A and B.
Bland-Altman plots of oxygen consumption (V̇O2) results as measured using the MGC Diagnostics CCM Express indirect calorimeter in the adult (A) and pediatric (B) range compared with the simulated values. Difference (%) = 100 × (measured value − set value)/[(measured value + set value)/2] versus average of the measured value and set value.
Discussion
We have reported the results of an in vitro experiment, testing 2 commonly used gas exchange measurement devices. Our study design allowed testing across a wide range of simulated pediatric and adult ranges for respiratory and metabolic values. Our results suggest that the indirect calorimeter and volumetric capnography devices were able to measure gas exchange values on average within 5% of each other, with mean bias and 95% CIs that were within the a priori defined clinically acceptable range compared with the reference method. The CCM Express demonstrated V̇O2 measurements that were within the limits of agreement. Furthermore, there was reasonable agreement between the 2 devices for V̇CO2 measurements. Our results support the use of portable gas exchange devices in adult and pediatric applications. However, their validation in the clinical setting must be further explored.
The Weir equation, which utilizes both V̇O2 and V̇CO2 to calculate energy expenditure, requires an indirect calorimeter. Recently, we have introduced an equation for estimation of energy expenditure that relies only on V̇CO2 measurements and may be used in the absence of an indirect calorimeter.14 Bedside V̇CO2 measurement devices are also used to titrate settings and assess changes in respiratory physiology in the ICUs. However, since devices to measure V̇CO2 (such as the NM3) have not been compared head to head with an indirect calorimeter, it is necessary to demonstrate their accuracy and agreement.
We have previously reported a similar simulation model to test the accuracy of a gas exchange device to measure gas exchange during noninvasive ventilation.15 Subsequently, the model has been modified for simulation of spontaneous breathing simulation as in the present study. Wines et al16 described the application of a similar simulation device to test the accuracy of an indirect calorimeter device over a wide range of gas exchange values. Indirect calorimeter accuracy and agreement have been explored in critically ill adult subjects.17,18 Black et al17 recently described the agreement of 3 techniques for gas exchange measurement and demonstrated that the bias between devices was acceptable, but the precision was relatively poor. Although the aforementioned studies address important questions for commercially available devices, neither the CCM Express nor the NM3 have been explored sufficiently. Therefore, our present study adds to the literature, and our observations will aid clinicians in determining the correct device for select cohorts.
Currently available gas exchange monitors use different methods for gas sampling and analyses. However, it was not the goal of the present study to specifically compare these methods, because it has been done previously.17,19–21 In our current study, the V̇CO2 measurements between the CCM Express indirect calorimeter and the NM3 volumetric capnography device were in agreement despite technological differences in flow and gas concentration measurement.
Although the values from both devices were within the a priori determined limits of ±20%, it must be noted that the CCM Express underestimated the gas exchange values by an average of ∼16% for V̇O2. This may result in unintended underprescription of energy, which could be clinically relevant in malnourished children. Table 1 and Figure 4 show this consistent bias. The reasons for this bias are not clear from our experiments. Despite a thorough evaluation for leaks in the experimental setup, a consistent leak during these experiments could result in a consistent bias such as the one described above, and this bias would affect results for all testing conditions with both devices. Future clinical studies must explore this and determine the source of the bias and the need to account for it when interpret the measurements by CCM Express.
There are important limitations to the present study that must be considered. First, the study was not conducted on human subjects. Rather, a metabolic simulator with precise control of respiratory parameters and gas exchange values was used. The present study attempts to limit the pitfalls of using an in vitro design to simulate not only gas exchange values, but also respiratory parameters that are typically observed in both adult and pediatric patients (including VT, breathing frequency, inspiratory and expiratory flow patterns, and inspiratory time). Further, the use of an in vitro system has important advantages over clinical testing due to the ability to precisely control respiratory parameters as mentioned above. However, the accuracy of the system components is an important consideration when interpreting the findings. For volume delivery with the ASL 5000, accuracy is ±2.0%, and for gas injection with the mass flow controller, the accuracy is ±0.8% of the measurement reading; the expected combined maximum error propagation of the accuracy of these components would be ±2.8%. Finally, because both devices yielded measurements below the reference method for both V̇O2 and V̇CO2, it is possible that the method of simulation introduced a systematic bias. However, great lengths were taken to ensure that the system was free of leaks, and all equipment was tested before use.
The next steps should include well-controlled clinical studies that discern the agreement between the 2 devices. Methods for transmitting continuous gas exchange monitoring data to electronic medical records would be desirable. The ultimate aim is to use validated gas exchange devices that provide accurate measurements of V̇O2 and/or V̇CO2, which can be applied at the bedside for daily titration of nutritional and respiratory therapies.
Conclusions
Currently available portable gas exchange monitors demonstrated acceptable, albeit variable agreement with simulated V̇O2 and V̇CO2 values. The CCM Express and the NM3 measured V̇CO2 with small mean bias and acceptable limits of agreement in both the pediatric and adult range.
Footnotes
- Correspondence: Craig D Smallwood RRT, Division of Critical Care Medicine, Department of Anesthesiology, Pain, and Perioperative Medicine, MSICU Office, Bader 634, Boston Children's Hospital, 300 Longwood Avenue, Boston, MA 02115. E-mail: craig.smallwood{at}childrens.harvard.edu.
Mr Smallwood has disclosed a relationship with Hill-Rom Medical.
- Copyright © 2016 by Daedalus Enterprises