Abstract
BACKGROUND: Volume-controlled ventilation modes have been shown to reduce duration of mechanical ventilation, incidence of chronic lung disease, failure of primary mode of ventilation, hypocarbia, severe intraventricular hemorrhage, pneumothorax, and periventricular leukomalacia in preterm infants when compared with pressure limited ventilation modes. Volume-guarantee (VG) ventilation is the most commonly used mode for volume-controlled ventilation. Assist control, pressure-support ventilation (PSV), and synchronized intermittent mandatory ventilation (SIMV) can be combined with VG; however, there is a lack of knowledge on the superiority of each regarding clinical outcomes. Therefore, we investigated the effects of SIMV+VG and PSV+VG on ventilatory parameters, pulmonary inflammation, morbidity, and mortality in preterm infants.
METHODS: Preterm infants who were born in our hospital between 24–32 weeks gestation and needed mechanical ventilation for respiratory distress syndrome were considered eligible. Patients requiring high-frequency oscillatory ventilation for primary treatment were excluded. Subjects were randomized to either SIMV+VG or PSV+VG. Continuously recorded ventilatory parameters, clinical data, blood gas values, and tracheal aspirate cytokine levels were analyzed.
RESULTS: The study enrolled 42 subjects. Clinical data were similar between groups. PSV+VG delivered closer tidal volumes to set tidal volumes (60% vs 49%, P = .02). Clinical data, including days on ventilation, morbidity, and mortality, were similar between groups. Chronic lung disease occurred less often and heart rate was lower in subjects who were ventilated with PSV+VG. The incidence of hypocarbia and hypercarbia were similar. Interleukin-1β in the tracheal aspirates increased during both modes.
CONCLUSION: PSV+VG provided closer tidal volumes to the set value in ventilated preterm infants with respiratory distress syndrome and was not associated with overventilation or a difference in mortality or morbidity when compared to SIMV+VG. Therefore, PSV+VG is a safe mode of mechanical ventilation to be used for respiratory distress syndrome.
- mechanical ventilation
- lung inflammation
- preterm infants
- tidal volume
- ventilator-induced lung injury
- dysplasia
- bronchopulmonary
Introduction
Volume-guarantee (VG) ventilation is one of the volume-controlled ventilation modes in which the ventilator automatically adjusts inspiratory pressure to achieve desired tidal volume (VT). Volume-controlled ventilation modes were shown to reduce duration of mechanical ventilation, incidence of chronic lung disease, failure of primary mode of ventilation, hypocarbia, grade 3/4 intraventricular hemorrhage, pneumothorax, and periventricular leukomalacia in preterm infants when compared with pressure-limited ventilation modes.1 It may be applied with pressure-support ventilation (PSV), assist-control ventilation, or synchronized intermittent mandatory ventilation (SIMV), although no data favors one over the other.2
To date, studies comparing synchronized modes with VG have been crossover studies and have reported that assist-control+VG or PSV+VG ventilation modes produced more stable VT compared to the SIMV+VG mode during weaning without focusing on clinical morbidities.3–5 Nevertheless, it is not clear which ventilation mode should combine with VG. SIMV+VG supports a pre-set number of breaths with time-cycling, whereas PSV+VG supports each breath with flow-cycling method. The two specifications of PSV, support of each breath and flow cycling, have been shown to produce shorter duration of mechanical ventilation and weaning, and work load, respectively, and thus may have an additive effect over VG when compared to SIMV+VG.6–9 However, overventilation due to support of each triggered breath is a potential risk that can result in hypocarbia and air leaks.
We compared the ventilatory parameters, blood gases, and short- and long-term clinical outcomes together with pulmonary inflammation markers of preterm infants with respiratory distress syndrome who were ventilated with either PSV+VG or SIMV+VG.
QUICK LOOK
Current knowledge
Volume guarantee (VG) ventilation has proven positive clinical effects and can be combined with pressure support ventilation (PSV) and synchronized intermittent mandatory ventilation (SIMV). PSV+VG achieves more uniform tidal volumes during weaning when compared to SIMV+VG.
What this paper contributes to our knowledge
PSV+VG allowed for more breaths with closer tidal volume to the set value in mechanically ventilated preterm infants with respiratory distress syndrome and did not cause overventilation and hypocarbia. PSV+VG did not increase morbidity and mortality when compared to SIMV+VG. Similar pulmonary inflammation occurred in PSV+VG and SIMV+VG. PSV+VG is a safe and reasonable mode, and thus can be used more frequently for treatment of respiratory distress syndrome in preterm infants.
Methods
This randomized, controlled trial was conducted between June 2011 and December 2013 (NCT01514331) in the Level III Neonatal Intensive Care Unit at the Gazi University Hospital, Ankara, Turkey. University Ethics Committee approved the study. Written informed consent was obtained from the parents.
Study Design
Preterm infants who were born in our hospital between 24–32 weeks gestation and needed mechanical ventilation treatment for respiratory distress syndrome in the first 12 h of life after surfactant treatment were considered eligible. Exclusion criteria were major congenital anomaly, pulmonary disease other than respiratory distress syndrome, absence of spontaneous respiration, high-frequency oscillatory ventilation (HFOV) before conventional ventilation, and extubation within 6 h. Our unit's indications for HFOV were pulmonary hypertension, air leak syndromes, and failure of conventional ventilation. The mechanical ventilation treatment was provided by Babylog 8000plus (Draeger, Lubeck, Germany). Subjects were randomized by a blinded neonatology fellow to either SIMV+VG or PSV+VG using sealed and sequentially numbered envelopes.
Baseline clinical characteristics including gestational age, birthweight, gender, mode of delivery, small for gestational age, Apgar score, surfactant treatment at delivery room, antenatal corticosteroid administration, preterm premature rupture of membranes, and clinical chorioamnionitis were recorded.
Ventilatory data, hemodynamic parameters, and blood gas analyses values were recorded for 72 h if extubation, mode change, or death did not occur earlier. Two tracheal aspirate (TA) samples were collected. The first samples (TA-1) were collected at 6 h after surfactant administration, and the second samples (TA-2) were collected at the time that data recording was stopped. Entire clinical morbidities were documented. Primary outcome was VT measurements during ventilation.
Ventilatory Management and Data Transfer
Initial ventilatory settings were: VT = 4 mL/kg; PEEP, 5 cm H2O; and peak inspiratory pressure (PIP), 2–3 cm H2O higher than the minimum pressure to deliver target VT; and FIO2 to maintain a saturation of 90–95% and trigger sensitivity, with a maximum sensitivity of 1 for both groups, on the basis of our clinical experience and accepted literature approaches.9 Target blood gas values were pH > 7.20–7.25; PaCO2 45–55 mm Hg; and PaO2 60–80 mm Hg. The VT was increased to 6 mL/kg when needed. In the SIMV+VG group, the breathing frequency was set as 40–60 breaths/min, and inspiratory time was set at 0.30–0.36 s. In the PSV+VG group, the breathing frequency was set as a back-up frequency of 40 breaths/min, and inspiratory time was limited to 1.5 times higher than the observed spontaneous inspiratory time (maximum, 0.60 s). During the weaning process, trigger sensitivity for PSV+VG and breathing frequency for SIMV+VG were adjusted besides set VT and FIO2 for both groups.
The set VT, expiratory VT, PIP set value, working PIP, mean airway pressure, minute ventilation, breathing frequency (set and measured), inspiratory time (set and measured), trigger sensitivity, air leak, and FIO2 were recorded every 10 s from the RS232 output port of the ventilator and were exported with Babyview1 software (Draeger) into a spreadsheet (Microsoft Excel, Microsoft, Redmond, WA).
Data cleaning was done according to the following criteria before analysis:
Breaths with leak > 40%.
Breaths with unmeasured expiratory VT, expiratory VT < 10% or > 200% of set VT.
Working PIP < PEEP.
PEEP < 3 cm H2O.
Measured breathing frequency was 5 breaths/min or more below the set frequency and 20 breaths/min or more higher than the subject's frequency.
We calculated the ratio of expiratory VT to set VT in percentages for each recorded breath (expiratory VT/set VT × 100). This value was categorized as appropriate expiratory VT if between 80% and 120%, as low expiratory VT if < 80%, and as high expiratory VT if > 120%. The VT percentage value, working PIP, mean airway pressure, minute ventilation, and FIO2 were analyzed among groups after each parameter were averaged for each subject.
Clinical Outcome Measures
Respiratory Outcomes.
HFOV requirement, repeated surfactant treatment, extubation failure (re-intubation due to abnormal blood gas with FIO2 > 0.50 or ≥2 apneas needing positive-pressure ventilation or clinical respiratory distress), time to extubation, total days of mechanical ventilation, and noninvasive ventilation.
Hemodynamic Parameters and Blood Gas Analysis.
Heart rate (beats/min), breathing frequency (breaths/min), mean arterial blood pressure (mm Hg), SpO2 (%) measurements, and PaO2 and PaCO2 were recorded manually. Incidences of hypocarbia (PaCO2 < 30 mm Hg), hypercarbia (PaCO2 > 55 mm Hg), hypoxemia (PaO2 < 50 mm Hg), and hyperoxemia (PaO2 > 80 mm Hg) were calculated as n(of parameter)/N(total number of blood gas analysis) × 100.
Morbidities and Mortality.
Short-term morbidities included clinically important patent ductus arteriosus, pulmonary hemorrhage, pulmonary interstitial emphysema, intraventricular hemorrhage (≥grade 3). Long-term morbidities included retinopathy of prematurity (≥stage 2), necrotizing enterocolitis, periventicular leukomalacia, chronic lung disease (oxygen dependence at 36th weeks), and sepsis. Mortality was death in unit before discharge.
TA Collection and Cytokine Analysis.
Two samples (TA-1 and TA-2) were collected. The TA-1 samples were collected at 6 h after surfactant administration. The TA-2 were collected at 72 h if extubation, mode change, or death did not occur earlier. TA samples were obtained after introducing 1 mL/kg saline into trachea. After being centrifuged (10 min at 3,600 revolutions/min), supernatants were stored at −80°C until analyzed for 4 interleukins (IL-1β, IL-6, IL-8, and IL-10). Interleukins were measured with the enzyme-linked immunosorbent assay method (eBioscience, Bender MedSystems GmbH, Vienna, Austria). Interleukin levels were presented as pg/mg-protein (TA protein; Quantichrom Protein Assay Kit, BioAssay Systems, Hayward, CA).
Statistical Analysis
Statistical analysis was performed using SPSS, version 15.0 (SPSS, Chicago, IL). A P value < .05 was considered statistically significant. Numerical variables were expressed as median (interquartile range [IQR]) and analyzed with the Mann-Whitney U test. Categorical variables were expressed as numbers n/N (%) and analyzed using the chi-square test. The Wilcoxon test was performed for paired measurements of TA-IL. The main outcome was percentage of expiratory VT in the appropriate range. The assist-control+VG was shown to cause 50% more breaths within the target VT range compared to assist-control.10 Based on that, power analysis revealed 16 subjects in each group to detect a 25% difference between the ratio of VT within the target range between the 2 modes with 80% power and α of 0.05.
Results
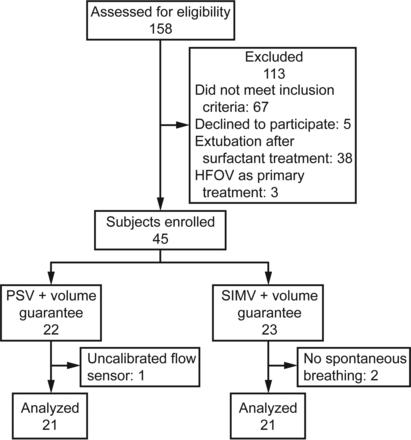
We assessed 158 patients (24–32 weeks gestation) for eligibility. Forty-two subjects were enrolled, divided evenly between the two study groups (subjects with birthweight ≤ 1,000 g: PSV+VG, n = 17; SIMV+VG; n = 15), and completed the study as shown in Figure 1. Baseline clinical characteristics were similar among groups (Table 1).
Flow chart. HFOV = high-frequency oscillatory ventilation, PSV = pressure-support ventilation, SIMV = synchronized intermittent mandatory ventilation.
Clinical Characteristics of Study Subjects
Ventilatory data, hemodynamic parameters, and blood gas analyses were evaluated for 42 h (IQR, 25–72) in the PSV+VG group and 52 h (IQR, 24–72) in the SIMV+VG group (P = .93).
Ventilatory Parameters
Data of 511,171 breaths were analyzed (PSV+VG: 240,658 breaths; SIMV+VG: 270,513 breaths). Percentage of excluded breaths (9% [IQR, 7–11%] and 10% [IQR, 8–11%], P = .20), leak (5% [IQR, 2–7%] and 4% [IQR, 1–6%]; P = .38), and set VT (4.1 mL/kg [IQR, 4.0–4.5 mL/kg] and 4.2 mL/kg [IQR, 4.0–4.8 mL/kg], P = .64) were comparable between the PSV+VG and SIMV+VG groups, respectively.
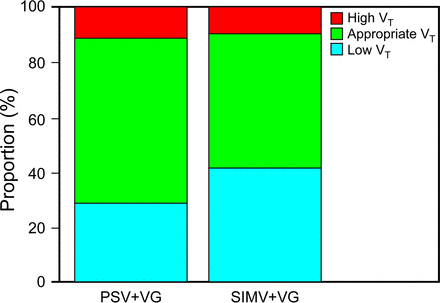
Infants in the PSV+VG group produced more breaths with appropriate VT and fewer breaths with low VT as shown in Figure 2. However, working PIP, mean airway pressure, minute ventilation, and FIO2 remained similar between groups as summarized in Table 2.
Distribution of categorized tidal volume percentages of subjects in SIMV+ volume guarantee and PSV+ volume guarantee groups (appropriate, low, and high VT) during the data recording period. Values refer to medians. Appropriate VT; P = .02, Low VT; P = .01. High VT is > 120% of set VT, appropriate VT = 80–120% of set VT, and low VT is < 80% of set VT.
Ventilatory Parameters During PSV+VG and SIMV+VG Ventilation
Clinical Outcomes
Entire clinical outcome measures are shown in Table 3. Four subjects in the SIMV+VG group died, and 2 subjects in the PSV+VG group died. Respiratory outcomes and morbidities were similar between groups. Three subjects in the PSV+VG group and 5 subjects in the SIMV+VG group required rescue HFOV treatment. No pneumothorax was observed. Chronic lung disease was observed less frequently in the PSV+VG group, although the difference was not statistically significant. None of the subjects with chronic lung disease received steroid treatment.
Respiratory Outcome, Important Morbidities, Hemodynamic Parameters, and Blood Gas Analysis Results
Hemodynamic Parameters and Blood Gas Analyses
Infants in the PSV+VG group had a slower heart rate than infants on SIMV+VG during the study period. Total blood gas analyses were 245 in the PSV+VG group and 230 in the SIMV+VG group. The number of analyses per subject [mean (IQR)] in the PSV+VG and SIMV+VG groups were 9 (6–15) and 8 (5–18), respectively. Hypocarbia occurred less frequently in the PSV+VG group (9%) than in the SIMV+VG group (20%), although the difference was not statistically different. Hypercarbia, hypoxia, and hyperoxia were observed to be similar between the 2 groups.
TA Cytokine Analyses
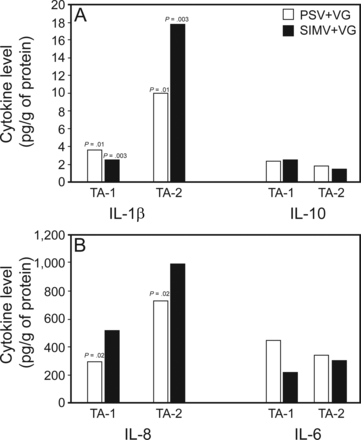
Of the 69 collected aspirates, 23 paired samples were sufficient for IL measurement (PSV+VG, 12; SIMV+VG, 11) due to failure to obtain supernatant. All TA-1 samplings were performed at 6 h, and median TA-2 sampling time was at 43 h (IQR, 32–72) in PSV+VG group and at 47 h (24–72) in the SIMV+VG group (P = .83). The IL-1β, IL-6, IL-8, and IL-10 levels were similar between both groups in TA-1 and TA-2 samples. IL-1β increased significantly in both groups. The IL-8 levels tended to rise in both groups, but the difference was statistically significant only in the PSV+VG group. The IL-6 and IL-10 levels remained stable. The TA-IL levels among groups are shown in Figure 3.
Cytokine levels in tracheal aspirates. Bars show medians. Wilcoxon test for paired measurements of interleukin levels in tracheal aspirates were statistically significant for IL-1β (P = .01) and IL-8 (P = .02) in PSV + volume guarantee group, and IL-1β (P = .003) in SIMV + volume guarantee group.
Discussion
In this study, we compared PSV+VG and SIMV+VG modes with regard to ventilation parameters, hemodynamic effects, blood gas values, clinical outcomes, and inflammatory responses. We observed that PSV+VG provided more appropriate expiratory VT values during the ventilation period compared to SIMV+VG, with no increase in adverse clinical effects.
It is hard to evaluate studies in the literature that compare different mechanical ventilation treatments because each treatment might differ from the other by more than one feature. The PSV+VG mode was most frequently compared to SIMV and was found to cause less postextubation atelectasis and required less blood gas analysis, whereas assessment of respiratory mechanics revealed conflicting results.11–14 However, these two modes are different from each other with regard to cycling (flow/time), control measure (volume/pressure), and supported number of breaths (all triggered/pre-set number).
Three previous short-term crossover trials assessed these 2 modes in stable preterm infants during weaning.3–5 Therefore, we compared PSV+VG to SIMV+VG in terms of clinical outcomes and pulmonary inflammation, in addition to ventilatory data monitored for longer periods of time. We found more appropriate VT values were delivered during PSV+VG than SIMV+VG. Because PSV+VG supports every triggered breath, whereas SIMV+VG supports only a set number of breaths, which affects the number of volume-guaranteed breaths, unsupported breaths therefore have VT values lower than the targeted range. In our study, higher minute ventilation was produced during PSV+VG ventilation than during SIMV+VG ventilation, possibly due to the delivery of more appropriate VT values, although the difference was not statistically significant. Short-term crossover trials reported working PIP, breathing frequency, and minute ventilation similar to our findings, while mean airway pressure results were inconsistent between PSV+VG and SIMV+VG. In our study, we observed that mean airway pressure was similar between groups.4–5 These conflicting results show the importance of individual variances and the difficulty of assessment of ventilatory parameters. We observed lower heart rate in PSV+VG group compared to SIMV+VG group, which is in line with the results of Abubakar and Keszler.3 Lower heart rate might be the result of total synchrony of patient with the ventilator, which is a feature of flow cycling.7 Hypocarbia, hypercarbia, and hyperoxia have been shown to contribute to the development of periventricular leukomalacia, hemorrhage, and retinopathy of prematurity, respectively.15–19 Lowest or highest arterial carbon dioxide or oxygen levels did not differ in either mode, making each equally reliable with regard to prevention of extreme values. Our weaning strategy in PSV+VG was based on decreasing trigger sensitivity, which may have contributed to the prevention of hypocarbia during PSV+VG. As trigger sensitivity is decreased, the number of breaths exceeding threshold would decrease, thus decreasing the number of supported breaths. The requirement for HFOV and the need for repeated surfactant treatment, incidence of patent ductus arteriosus, time to extubation, extubation failure, days of mechanical ventilation, and noninvasive ventilation were similar in both groups. It is worth noting that the high antenatal steroid rate in PSV+VG group, although not statistically significant, may have affected the severity of respiratory distress and masked probable differences in terms of ventilatory effectiveness.
Ventilation-induced lung injury is an important complication of mechanical ventilation, and cytokine response in TA of ventilated newborns has been considered as its biomarker.20 We observed elevation of IL-1β levels over time in both groups, supporting previous findings that conventional ventilation increases IL-1β levels.21 Most studies compared pulmonary inflammation either to evaluate effects of HFOV or VG ventilation.21–23 In the limited number of TA samples tested in our study, there was no substantial differences between cytokines with either VG ventilation method.
The major feature of our study is that we reported clinical outcomes regarding PSV+VG or SIMV+VG ventilation. Evaluation of ventilatory parameters during a long period of ventilation is another positive aspect. A major limitation of our study is the small sample size, which was due to increased use of noninvasive ventilation as our institution's respiratory support strategy. Although we have been able to document the VT difference between groups, our study is not powered enough to document the difference in mortality and incidence of clinical outcomes including chronic lung disease. Likewise, different antenatal steroid administration ratios in the groups might have influenced respiratory outcomes. Furthermore, insufficient paired TA samples restricted the assessment of inflammation.
Conclusions
Our study supports the finding that PSV+VG allows for more stabilized breaths by providing more appropriate VT in ventilated preterm infants with respiratory distress syndrome. However, breathing frequency and minute ventilation were similar between groups, while heart rate was lower in the PSV+VG group. Overventilation with hypocarbia or hyperoxia were similar between the groups. Chronic lung disease occurred less with PSV+VG but difference was not statistically significant. While our results could not be generalized regarding the limitations we discussed, we can suggest that PSV+VG is a safe mode to be used for treatment of respiratory distress syndrome in preterm infants. Further studies investigating the clinical outcomes of different ventilation methods with VG involving larger populations are warranted.
Acknowledgments
We would like to thank to Nuray Duman, Professor of Pediatrics (Dokuz Eylül University, Division of Neonatology) for providing the Babyview1 program.
Footnotes
- Correspondence: Sezin Unal MD, Etlik Zubeyde Hanım Women's Health Teaching and Research Hospital Yeni-Etlik Caddesi, 06010 Etlik, Kecioren, Ankara, Turkey. E-mail: sezinunal{at}gmail.com
Dr Unal presented a version of this paper as a poster at the 5th Congress of the European Academy of Paediatric Societies, held October 17–21, 2014, in Barcelona, Spain.
This study was funded by Gazi University Research Fund (Fund number: 01/2011-68).
Clinical Trial Registration: NCT01514331
See the Related Editorial on Page 1615
- Copyright © 2017 by Daedalus Enterprises