Abstract
BACKGROUND: Noninvasive ventilation (NIV) has proven to be useful in the management of children with acute respiratory failure as a result of acute lower respiratory infection. Despite this, evidence addressing the initiation and/or discontinuation criteria of NIV in children remains limited. The objective of this study was to evaluate the usefulness and clinical impact of an NIV protocol in hospitalized children with acute respiratory failure because of acute lower respiratory infection.
METHODS: A randomized controlled clinical trial was carried out among subjects admitted during the winter season at Hospital Josefina Martinez between May and October of 2013. Inclusion criteria were age 3 months to 2 y, diagnosis of acute lower respiratory infection and requiring NIV according to a Modified Wood Scale score of ≥ 4 points. Subjects were randomized to NIV management according to medical criteria (control group) or to protocolized management of NIV (protocol group). Hours of NIV, hospital stay, and supplemental oxygen use after discontinuation of NIV, severity changes after NIV initiation, respiratory symptoms, and proportion of intubations were considered as events of interest.
RESULTS: A total of 23 subjects were analyzed in the control group and 24 were analyzed in the protocol group. Hours of hospital stay, NIV, and supplemental oxygen post-NIV were not significantly different between groups (P = .70, .69, and .68, respectively). There were also no differences in intubation rate (3 of 29 for the control group and 2 of 31 for the protocol group). For the total sample there was a statistically significant decrease in the Modified Wood Scale score after 1 h of NIV (P < .001). A similar result was observed when performing a stratified intragroup analysis.
CONCLUSIONS: We observed that the implementation of an NIV management protocol that integrates initiation and discontinuation criteria for NIV is feasible. However, its use showed no advantages over a non-protocolized strategy.
Introduction
Acute respiratory failure as a result of acute lower respiratory infection is one of the most frequent causes of morbidity and mortality in pediatric patients, leading to an important public health concern.1–5 When these patients are admitted to a hospital, they often receive noninvasive ventilation (NIV) because of its benefits in both children and adults with acute and chronic respiratory diseases.3,5,6–8 NIV has been shown to decrease respiratory muscle workload, optimize alveolar recruitment, and minimize dynamic hyperinflation. In addition, there is evidence that NIV may improve ventilation and oxygenation in patients with acute lower respiratory infection, even reducing the need for intubation.6–11 However, despite the benefits of NIV, the development of specific criteria for its initiation and discontinuation in the pediatric population is limited mainly because of poorly detailed ventilatory strategies and heterogeneity of patients and their pathologies.6,11–13
The use of standardized criteria and guidelines in clinical practice constitutes a safe, uniform therapeutic approach capable of preventing potential harm to the patient. The use of protocols has been studied mostly in the context of invasive mechanical ventilation.14 In 2002, Randolph et al14 studied the effect of a weaning protocol on time of ventilator liberation and extubation failure rates in 182 children requiring invasive mechanical ventilation, showing no difference between conventional management and a planned strategy. However, Foronda et al15 evaluated the effect of a weaning protocol on 294 children, showing that having subjects undergo a daily and rigorous evaluation of a spontaneous breathing trial significantly reduces time to extubation compared with the standard management group (3.5 and 4.7 d, respectively). Additionally, the use of a computerized weaning protocol in children undergoing invasive mechanical ventilation demonstrated a significant decrease in the duration of invasive mechanical ventilation compared with standard management and medical judgment with 21 and 90 h, respectively.16
Based on the described data above, the use of protocolized ventilatory support has been shown to be beneficial in children undergoing invasive mechanical ventilation. However, evidence regarding protocolized initiation and discontinuation of NIV in children is limited and remains uncertain. In fact, Valenzuela et al4 demonstrated through an epidemiological study that 55% of pediatric ICUs in Chile lack formal criteria for initiation and discontinuation of NIV, and that 70% of these units do not have a corresponding clinical protocol. The main objective of this study was to evaluate the usefulness and clinical impact of a NIV protocol in hospitalized children with acute respiratory failure as a result of acute lower respiratory infection.
QUICK LOOK
Current knowledge
Noninvasive ventilation (NIV) has proven to be useful in the management of children with acute respiratory failure as a result of acute lower respiratory infection. The use of standardized criteria and guidelines in clinical practice constitutes a safe, uniform therapeutic approach capable of preventing potential harm to the patient. However, evidence regarding protocolized initiation and discontinuation of NIV assistance in children is limited and remains uncertain.
What this paper contributes to our knowledge
Implementation of a NIV management protocol including initiation and discontinuation criteria was feasible and safe in children hospitalized with acute respiratory failure. A protocolized strategy was not able to improve hospital length of stay, duration of NIV, or supplemental oxygen duration post-NIV compared with a non-protocolized management.
Methods
Subjects and Study Design
A randomized, controlled clinical trial was carried out among subjects admitted during the winter season at Hospital Josefina Martinez between May and October of 2013. The subjects included were ages 3 months to 2 y with acute respiratory failure because of acute lower respiratory infection, diagnosed by the presence of fever (defined as temperature > 38°C) and cough > 3 d, and at least one of the following signs: fine crackles or wheezing on pulmonary auscultation, chest radiograph with interstitial, alveolar infiltration, or consolidation and requiring NIV. The latter was defined according to a Modified Wood Scale score of ≥ 4 points.2,9,16 The Modified Wood Scale has been used to discriminate respiratory distress in patients with acute respiratory failure, including oxygenation, pulmonary ventilation, respiratory muscle workload, lower-airway obstruction, and changes in consciousness.10,17–20 Patients with previous NIV use and those with a history of chronic lung disease, oxygen dependence, and neuromuscular disease were excluded.
Informed consent was obtained from parents before recruitment. The study was approved by the Research Ethics Committee of the South Eastern Metropolitan Health Service of Santiago, Chile.
Randomization
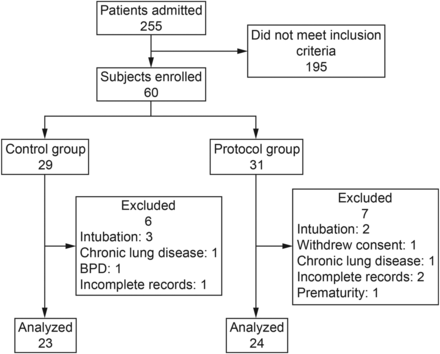
Subjects included in the study were randomized to NIV according to usual medical criteria (control group) or to protocolized management (protocol group) (Fig. 1). Simple randomization was performed with the statistical program Epidat 4.0. Sequence distribution was delivered through a list with 60 numbers already randomized to the control group or protocol group. Thus, as the subjects were admitted to Hospital Josefina Martinez and met study inclusion criteria, they were assigned a number determining their group. Enrollment was guided by respiratory therapists according to a described scheme, and a resident physician indicated the need for ventilatory assistance as described previously. The intervention was blinded to nursing staff.
Flow chart. BPD = bronchopulmonary dysplasia.
NIV Protocol
The Hospital Josefina Martinez protocol of ventilatory support was based on NIV initiation and discontinuation criteria, with maintenance of continuous support until its definitive suspension without pressure drops during the first 24 h.
The protocol included 7 assessment stages as shown in Figure 2: (1) initial assessment to decide the use of NIV (resident physician); (2) assessment after 1 h of NIV (resident physician or respiratory therapist); (3) assessment after 3 h of NIV (resident physician or respiratory therapist); (4) assessment after 24 h of NIV (resident physician or respiratory therapist); (5) assessment after 4 h of a pressure drop to inspiratory positive airway pressure 10/expiratory positive airway pressure 6 cm H2O (resident physician or respiratory therapist); (6) assessment after 4 h of a mode change to a continuous positive airway pressure of 6 (resident physician or respiratory therapist); (7) assessment after 4 h of discontinuing NIV (resident physician or respiratory therapist).
Algorithm for noninvasive ventilation (NIV) protocol. Modified Wood Scale (MWS) and index between oxygen saturation and inspired oxygen fraction (SF) were used for management decisions. NIV parameters were considered through changes in inspiratory positive airway pressure (IPAP), expiratory positive airway pressure (EPAP), inspiratory time (TI), and backup breathing frequency (f).
At all stages of the protocol, Modified Wood Scale scores, ventilatory parameters (mode, inspiratory positive airway pressure, expiratory positive airway pressure, continuous positive airway pressure, inspiratory time [Ti] and backup breathing frequency) and SpO2/FIO2 were recorded.21,22 Modifications of NIV were made according to physician criteria if subjects experienced a detrimental clinical course. The latter was defined as an increase in the Modified Wood Scale score from the last score recorded. For comparative analyses between groups, assessments 1 and 2 were used. These are the only common points of evaluation, given the nature of the control group intervention.
Events of Interest
Primary outcomes were considered as: (1) NIV duration measured in hours; (2) duration of hospital stay measured in hours and; (3) supplemental oxygen duration after discontinuation of NIV measured in hours. Events of interest were categorized according to their distribution, considering the 50th percentile as the cutoff. Thus, new variables emerged including prolonged hospital stay of > 167 h, prolonged NIV use of > 87 h, and prolonged supplemental oxygen duration of > 22 h. Secondary outcomes were Modified Wood Scale changes (assessment stages 1 and 2 for both groups, and additionally assessment stages 4 and 6 for the protocol group), intubation rate, and SpO2/FIO2 change.
Statistics
Considering patients previously admitted to our hospital during the winter seasons, a 5% error margin, a statistical significance when P < .05, and a power calculation of 80%, a sample size of 60 subjects was estimated, with 29 and 31 subjects to control and protocol groups, respectively, after randomization. The Stata 12.0 statistical program (Stata, College Station, Texas) was used to analyze the pre- and post-intervention data in both groups. Frequencies, median, interquartile range, mean, and SD were used for the descriptive analysis, as appropriate. Age, severity of respiratory distress (Modified Wood Scale), sex, and diagnosis on admission were considered. In addition, the differences between them were analyzed with t test, chi-square, analysis of variance, Mann-Whitney, and Wilcoxon tests, as appropriate. Differences in the distribution of events of interest (duration of hospital stay, duration of NIV, and supplemental oxygen use of after discontinuation of NIV) between study groups were evaluated using the Mann-Whitney test. For each dichotomized event of interest, the odds ratio (and 95% CI) was estimated to evaluate the association with the intervention.
Modified Wood Scale and SpO2/FIO2 changes at different assessment stages were analyzed by paired t test and Wilcoxon signed-rank test, as appropriate. P values were considered for statistical significance.
Results
Patients were recruited during the winter season between May and October in 2013. A total of 255 patients were admitted to Hospital Josefina Martinez. The first 60 children (sample size estimated) who met the inclusion criteria were included systematically in the study. Of these subjects, 5 (8.3%) were excluded because of intubation, 4 (6.7%) because of the presence of comorbidities discovered during the course of the study, 2 (3.3%) were not considered for analysis because of incomplete records, 1 (1.7%) was withdrawn because of a lack of informed consent and another because of an erroneous description of age. Of 60 subjects included in the study, only 47 (78.3%) were analyzed, 23 in the control group and 24 in the protocol group (Fig. 1).
Demographic characteristics of the sample are described in Table 1. Statistically significant differences were found between groups regarding severity of respiratory distress and sex (P < .001 and P = .02, respectively). These variables were not associated with the events of interest (data not shown), ruling out the need for a stratified analysis.
Baseline Characteristics of Sample
No significant differences were found in duration of hospital stay, duration of NIV, and supplemental oxygen duration after NIV discontinuation between study groups (Table 2). Similarly, there were no differences in the rate of intubation; 3 of 29 (10.3%) for the control group and 2 of 31 (6.5%) for the protocol group. However, there was a statistically significant decrease in severity score after 1 h of NIV when all samples were analyzed (P < .001) (see Table 3). Additionally, no association was found between study groups for prolonged duration of hospital stay, prolonged duration of NIV, and prolonged supplemental oxygen therapy after NIV discontinuation was found (P = .47, .88, and .10, respectively) (Table 4).
Comparison of Impact Indicators by Intervention
Secondary Outcomes by Treatment Group
Association Between Intervention and Events of Interest
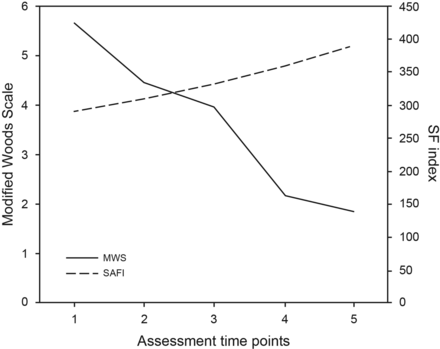
Trends in respiratory failure severity were evaluated through Modified Wood Scale and SpO2/FIO2 changes in the protocol group (Fig. 3). Modified Wood Scale scores showed a significant decrease between baseline and 1 h (P < .001), between 3 and 24 h (< .001), and within 24 h after NIV start. The SpO2/FIO2 showed a significant increase from 313.0 ± 14.9 to 389.8 ± 68.8 (P = .03) after 24 h of NIV.
Trends in respiratory failure severity in the protocol group were evaluated through changes in Modified Wood Scale (MWS) and the index between oxygen saturation and inspired oxygen fraction (SF). The assessment time points considered were (1) baseline, (2) 1 h, (3) 3 h, and (4) 24 h post-initiation of noninvasive ventilation, and (5) 4 h post-change of mode to CPAP before discontinuation.
Discussion
The results of this study seem to reinforce the positive effect of NIV in children with acute lower respiratory infection reported by other studies,5 showing a decrease in severity (evidenced by the drop in Modified Wood Scale score) and increased oxygenation (evidenced by an increase in SpO2/FIO2) after initiation of ventilatory support. However, it is important to note that the entire study group received ventilatory support, making it difficult to assume that the improvement found was only because of NIV use.
In our study, the proportion of total intubations for both groups was 17%, which is 11% lower than that observed by Yañez et al6 in subjects who received NIV (28%). This result could be explained by the degree of severity at the moment of NIV initiation, where the criterion established by Yañez et al6 considered a Modified Wood Scale score of > 5 (range of severity on admission of 5–8), while in our study, patients were considered with a Modified Wood Scale score of ≥ 4 (range of severity on admission of 4–7.5), suggesting that early ventilatory assistance could be a key aspect to avoid patient fatigue, disease progression, and the need for invasive mechanical ventilation through an endotracheal tube.
Analyzing the duration of hospital stay in the total sample (equivalent to 8.2 d), found that this was lower than reported in the literature for other subjects receiving NIV (10.4 d, according to Yañez et al6). As we mentioned above, the Modified Wood Scale score of ≥ 4 inclusion criteria could explain this result as well; nevertheless, it is necessary to consider that many patients admitted to our center were transferred from other hospitals where they received initial management between 1 and 3 d.
The main findings of this study suggest that there would be no advantage to using a protocolized strategy versus conventional management when comparing duration of hospital stay, duration of NIV, and supplemental oxygen duration after discontinuation of NIV. These results are in line with the experience of Randolph et al14 in pediatric subjects undergoing invasive mechanical ventilation, but differs from that found by Foronda et al,15 who evaluated the effect of a daily spontaneous breathing trial during invasive mechanical ventilation and found differences between a protocolized strategy versus conventional management.
It is worthy of note that the Randolph et al14 and Foronda et al15 studies were performed in subjects receiving invasive mechanical ventilation, which presents important differences in terms of frequency of monitoring, ventilatory mode, and risks associated with the use of an artificial airway other than NIV, making comparison difficult. As in our study, Randolph et al14 integrated a protocolized management from initiation of ventilatory support until its discontinuation. However, other studies demonstrating a positive result following the use of a protocolized strategy have been focused only on weaning management without considering the initial management.13,16,23–25
Although the main events of interest were not statistically different between study groups, the observation that there was one fewer intubated child in the protocol group should be highlighted within the clinical context. There was a significantly higher severity score in the protocol group, suggesting some advantage of a protocolized strategy over conventional management in the context of higher severity. However, this must be determined by further studies.
Our study provides a novel experience for the management of these patients because our protocol included initiation and discontinuation criteria for NIV use, which have been scarcely studied in children with acute lower respiratory infection requiring NIV.6,8
Several limitations are found because of the nature of our study. Although interventions were blinded to nursing staff, the medical staff following the protocol could not be blinded to the intervention, making it possible to replicate strategies between groups (applying protocolized strategies to the control group) and masking the advantage of protocolization over standard management. Moreover, given that a protocolized strategy includes strict monitoring, we could not collect all data at the same assessment time points between groups, allowing a complete analysis only in the protocol group.
Conclusions
There is limited experience related to protocolized NIV strategies in children with acute lower respiratory infection. We observed that the implementation of a protocolized intervention, which integrates initiation and discontinuation criteria for NIV, is feasible. However, its use did not exhibit statistical advantages in terms of hospital stay, duration of NIV, and supplemental oxygen therapy. Future clinical studies incorporating a protocolized NIV strategy in pediatric patients are required to complement these findings.
Footnotes
- Correspondence: Yorschua Jalil PT, Department of Kinesiology and Respiratory Rehabilitation, Hospital Josefina Martinez, Avenida Camilo Henriquez 3691, Puente Alto, Santiago, Chile.
- Copyright © 2017 by Daedalus Enterprises