Abstract
BACKGROUND: Nasal prongs are frequently used to deliver noninvasive CPAP in bronchiolitis, especially in the youngest children. A helmet interface is an alternative that might be comparable to nasal prongs. We sought to compare these interfaces.
METHODS: We performed a prospective, randomized, crossover, single-center study in an 8-bed multidisciplinary pediatric ICU in a university hospital. Infants age <3 months who were consecutively admitted to the pediatric ICU during a bronchiolitis epidemic season and fulfilled inclusion criteria were recruited. Subjects were randomly allocated to receive CPAP via a helmet or nasal prongs for 60 min. The subjects were then placed on the other CPAP system for another 60-min period (helmet then nasal prongs [H-NP] or nasal prongs then helmet [NP-H]). Measurements were taken at 30, 60, 90, and 120 min. Failure was defined as the need for further respiratory support.
RESULTS: Sixteen subjects were included, with 9 in the H-NP group and 7 in the NP-H group. CPAP significantly reduced respiratory distress, showing no differences between the H-NP and NP-H groups in terms of improving the Modified Wood's Clinical Asthma Score from 4.8 ± 1 to 3 ± 0.9 and 2.7 ± 1.7 points at 60 min and 120 min in the H-NP group, respectively, and from 4.2 ± 0.9 to 2.8 ± 0.9 and to 2.9 ± 0.9 at 60 min and 120 min, respectively, in the NP-H group. Sedatives were used in only 3 subjects (2 in the NP-H group, P = .77). The failure rate was similar in both groups (3 of 9 subjects vs 3 of 7 subjects, P = .70). No significant differences were seen for heart rate, breathing frequency, FIO2, or transcutaneous oxygen saturation response.
CONCLUSIONS: Our results suggest that CPAP delivered by nasal prongs and CPAP delivered by helmet are similar in terms of efficacy in young infants with acute bronchiolitis.
Introduction
Bronchiolitis is the leading cause of admission in children younger than 1 y worldwide. No effective treatments are available, apart from hydration, oxygen, and upper airway suctioning if needed.1 Noninvasive CPAP has been proven to be effective as a respiratory support in moderate to severe bronchiolitis2 to avoid invasive ventilation by decreasing respiratory muscles upload3 and maintaining patency of the lower airways. CPAP is usually delivered via nasal prongs or a nasal mask.2,4 However, the effectiveness of nasal CPAP might be limited due to air leakage through the child's mouth, airway obstruction from secretions, or nasal injury.5,6
A helmet interface has been proposed as an alternative to nasal administration of CPAP in infants and preschool children with acute respiratory failure,7–12 in preterm infants,13 and also in infants suffering bronchiolitis in conjunction with heliox.14 Furthermore, helmet-delivered CPAP has shown increased tolerance and increased effectiveness when compared to face mask in a crossover study7 and in infants with acute respiratory failure.8 A recent multi-center randomized controlled trial in respiratory syncytial virus bronchiolitis also described better tolerance and less need of sedation when using the helmet compared with the face mask, although intubation rates did not differ.9 Helmet CPAP has also been compared to high-flow nasal cannula oxygenation in mild to moderate respiratory distress in children < 2 y old, with similar results reported.12
Small infants with bronchiolitis requiring respiratory support have been treated in our pediatric ICU with CPAP by means of the helmet interface and nasal prongs, as in other studies.7,12,14–17 The objective of this study was to compare the effectiveness of CPAP delivered via a helmet or via nasal prongs in terms of clinical assessment of the work of breathing.
QUICK LOOK
Current knowledge
Bronchiolitis is one of the most substantial health burdens for infants worldwide. Management is supportive, focusing on maintaining oxygenation and hydration of the patient. CPAP has been proven to be effective as a respiratory support in moderate to severe bronchiolitis. There are several interfaces available to deliver CPAP.
What this paper contributes to our knowledge
Infants with bronchiolitis were randomized to receive CPAP via one interface and then another (helmet followed by nasal prongs, or nasal prongs followed by helmet). Sedation use, CPAP duration, CPAP failure rates, and clinical variables were similar between groups. Both methods of CPAP delivery were found to be equally effective at reducing respiratory distress.
Methods
Subjects
Subjects were consecutively admitted infants under 3 months of age diagnosed with bronchiolitis, with a Modified Wood's Clinical Asthma Score (M-WCAS) ≥ 4, venous PCO2 ≥ 60 mm Hg, or SpO2 < 92% despite nebulized adrenalin for at least 1 h (modified from Martinón-Torres16). The diagnosis of bronchiolitis was made if a child with a previous upper respiratory infection became tachypneic and exhibited prolonged expiratory time, wheezing, rales, respiratory accessory muscle activation, and hyperinflation on chest radiograph.18
Exclusion criteria included cardiorespiratory arrest, neurological impairment with inability to maintain airway patency, hemodynamic instability, inability to manage secretions despite frequent suctioning, undrained pneumothorax, cyanotic congenital heart disease, severe pulmonary condition, and immune deficiencies.
This research project was approved by the Research Ethics Committee of Hospital Universitario Central de Asturias. Written informed consent was obtained from subjects' parents or guardians.
Definitions
CPAP failure was defined as the need to intubate the infant or switch to noninvasive ventilation (NIV) due to worsening clinical condition. Major adverse events were defined as the appearance of hemodynamic instability, severe arrhythmias, barotrauma (eg, pneumothorax, pneumomediastinum, or massive subcutaneous emphysema), and hypercapnic coma. Other adverse events considered to be mild or moderate were pressure sores, gastric distention, and conjunctivitis.
Study Protocol
Subjects were kept in a semirecumbent position, and suctioning of secretions was performed as needed. The first subject was randomly allocated to receive CPAP either by means of a helmet interface (CaStar; Starmed, Mirandola, Italy) or nasal prongs (Infant Flow driver, Viasys, Conshohocken, Pennsylvania) for 60 min. Subsequent subjects included in the study were alternatively allocated to receive helmet-delivered CPAP or CPAP via nasal prongs in the first period. After the first 60 min, a careful suctioning of secretions was performed, and subjects were then placed on the other CPAP system for another 60-min period. Measurements were taken at baseline and at 30, 60, 90, and 120 min. The group in which CPAP was initially delivered by helmet followed by nasal prongs was named the H-NP group. The other cohort was named the NP-H group.
After this 120-min period, CPAP was maintained with the system used in the second phase of the study for as long as considered necessary by the clinician in charge. If a subject's clinical condition worsened at any time, such as with increasing respiratory distress, hypoxemia, hypercapnia, or exhaustion, the attending physician decided whether to change to NIV by means of a face mask or to intubate the subject.
CPAP Equipment
When using the helmet interface, CPAP was delivered via a noninvasive ventilator (CF 800; Dräger, Lübeck, Germany) connected to an air-oxygen port at a flow of 30 L/min with the intent to avoid rebreathing.19 A soft cushion was placed inside the helmet to avoid pressure sores on the neck or occipital region. When using nasal prongs, the CPAP system was the Infant Flow driver (Viasys).
Initial optimal CPAP was set to maintain SpO2 > 92% using the lowest FIO2. Minimum CPAP was set at 5 cm H2O. A cascade-type heated humidifier was used in all cases (MR850, Fisher & Paykel, Auckland, New Zealand). A gastric tube was inserted in all subjects to avoid gastric distention and to feed a subject when possible.
If a subject appeared distressed, intravenous midazolam boluses were administered (0.05–0.10 mg/kg), and, if needed, a continuous perfusion was started (0.05–0.10 mg/kg/h). No subject received any nebulized medications, systemic corticosteroids, or caffeine. The need for sedation and the occurrence of adverse events was also recorded. A nasopharyngeal swab specimen was tested for respiratory syncytial virus in all cases.
End Points
The primary end point was to compare the clinical response of the H-NP group with that of the NP-H group in terms of the M-WCAS. Secondary end points included the comparison of other variables (ie, heart rate, breathing frequency, SpO2, and FIO2) and the variation percentage to initial value (VPIV) of these figures between both groups. Other secondary end points were the comparison of CPAP failure, the occurrence of adverse events, and the use of sedatives between the groups.
Statistical Analysis
Following Grizzle's study, we analyzed the possible carryover effect, as well as the period effect.20 The normal distribution of variables was assessed using the Shapiro-Wilk test. A paired t test was performed to analyze differences between both treatment groups. The evolution of variables was measured at each moment as VPIV. Given a moment 1 and a variable A, the variation percentage of variable A at moment 1 (AVP1) was measured according to the formula AVP1 = 100 × (A1 − A0)/A0, where A0 is the initial value of variable A.
Sample size was calculated based on M-WCAS and was computed to detect mean differences between helmet and nasal CPAP patients of over 15% with a power of 0.8 (1 − type-2 error) and a type-1 error of 0.05. Based on a previous pilot study, we assumed that M-WCAS ranged from 10% to 20%, resulting in a minimum sample size of 7 per group.
Results
A total of 17 subjects fulfilled the inclusion criteria and completed the crossover study period; 1 subject was excluded because he was diagnosed with cystic fibrosis after admission. No subject required intubation or switching to NIV during the 2-h study period, and no apneas requiring intervention were registered (3 hypopneas in the NP-H group, and 2 hypopneas in the H-NP group). Subject characteristics are shown in Table 1.
Subject Characteristics
In the H-NP group, 1 subject showed poor tolerance of the nasal prongs used in the second part of the study and was switched back to helmet-delivered CPAP nearly 3 h later. Similarly, 1 subject in to the NP-H group was changed back to nasal-prong CPAP after the crossover study due to poor tolerance of the helmet. No adverse events occurred during the crossover phase. The level of CPAP delivered did not differ between groups with the following values (in cm H2O): 6.1 ± 1.2 at 30 min, 6.2 ± 1 at 60 min, 6.2 ± 1.1 at 90 min, and 6.2 ± 0.9 at 120 min.
Two subjects, both in the H-NP group, required intubation. One developed severe hypoxemia due to pneumothorax 6 h after the crossover phase while receiving nasal prong CPAP. The other subject was changed back to the helmet interface 2 h after the crossover phase due to increasing distress with the nasal prongs, but 3 h later he was placed on NIV and was finally intubated 8 h later. Apart from these 2 subjects, who were eventually intubated, another 4 subjects experienced increasing respiratory distress despite CPAP treatment and were placed on NIV, obviating the need for intubation; 3 subjects belonged to the NP-H group and 1 subject was in the H-NP group. Thus, CPAP failure occurred in 3 cases in each group. Figure 1 presents the distribution of subjects in the study and the clinical outcome in each group. Apart from the previously mentioned pneumothorax, no other adverse events were registered. The duration of CPAP did not differ between the 2 groups. All of the subjects of the study survived.
Flow chart. NIV = noninvasive ventilation.
Clinical Score
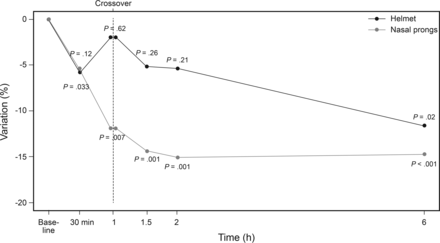
M-WCAS improved significantly from baseline in both the H-NP group and the NP-H group (Fig. 2). In the H-NP group, M-WCAS fell from 4.8 ± 1 to 3 ± 0.9 at 60 min (P < .001), and to 2.7 ± 1.7 at 120 min (P < .001). In the NP-H group, M-WCAS decreased from 4.2 ± 0.9 to 2.8 ± 0.9 at 60 min (P < .001), and to 2.9 ± 0.9 at 120 min (P < .001). Figure 2 presents the evolution of the VPIV of M-WCAS during the crossover phase and for 6 h in both groups. No statistically significant differences were detected in M-WCAS improvement between the 2 groups.
Evolution of percentage variation of the M-WCAS. Note the similar decrease in both groups in the first part of crossover study (30 min and 60 min). P values show changes of variation percentage of M-WCAS from initial value. No differences were found between the groups. Helmet = helmet CPAP followed by nasal prong CPAP. Nasal prongs = nasal prong CPAP followed by helmet CPAP. M-WCAS = Modified Wood's Clinical Asthma Score.
Heart Rate
In H-NP group, the heart-rate VPIV did not reach statistical significance until 6 h. In the NP-H group, heart-rate VPIV significantly decreased from 30 min (P = .033), and this decrease was maintained throughout the first 6 h. However, there were no significant differences between the 2 groups (Fig. 3).
Changes in percentage variation of heart rate. P values show variation percentage of heart rate from initial value. No statistically significant differences were found between the groups. Helmet = helmet CPAP followed by nasal prong CPAP. Nasal prongs = nasal prong CPAP followed by helmet CPAP.
Respiratory Rate
Clinical evolution of breathing frequency did not differ at any moment between the study groups (Fig. 4). In the H-NP group, VPIV was already statistically significant at 90 min (P = .033), while in the NP-H group it did not achieve statistical significance until 6 h (P = .01). In the NP-H group, there was a significant increase in the variation percentage of breathing frequency at 90 min (after switching to helmet-delivered CPAP at 60 min).
Changes in percentage variation of breathing frequency. P values show variation percentage of breathing frequency from initial value. No statistically significant differences were found between the groups. Note the significant increase in variation percentage of breathing frequency at 90 min in the helmet group. Helmet = helmet CPAP followed by nasal prong CPAP. Nasal prongs = nasal prong CPAP followed by helmet CPAP.
FIO2 and SpO2
We found no significant percentage variations to initial values for FIO2 and SpO2 in both groups. No differences in FIO2 or SpO2 were found between treatment groups either.
Use of Sedatives
No differences were found between 2 groups. During the 2-h period, only 3 subjects required sedatives (all during the first hour): 2 subjects in NP-H group (1 single bolus and 1 bolus plus continuous infusion of midazolam), and 1 subject in the H-NP group (bolus plus continuous infusion of midazolam).
Discussion
To our knowledge, this is the first randomized study comparing helmet-delivered CPAP and CPAP delivered via nasal prongs in young infants with moderate to severe respiratory syncytial virus bronchiolitis. The results reported here suggest that the helmet interface might be an alternative to nasal prongs to treat bronchiolitis in infants up to 3 months of age. The 2 groups were comparable in terms of severity, hypercapnia, and baseline vital signs. In addition, the crossover design of the study minimized the possible bias of age.
In our study, both CPAP systems seemed to be able to significantly decrease the clinical score shortly after CPAP initiation. This relief in the work of breathing was maintained throughout the study time in most cases (Fig. 2). Unsurprisingly, heart rate and breathing frequency significantly decreased after CPAP initiation, as described in previous studies.9,15,21 The slight differences seen in heart rate and breathing frequency evolution may be explained by the sample size. However, this response did not significantly differ between groups (Figs. 3 and 4).
The effectiveness of CPAP in moderately severe bronchiolitis has been reported in several studies. In a randomized controlled trial, Thia et al2 showed increased effectiveness in reducing PCO2 when CPAP was delivered initially in comparison with initial spontaneous breathing. Cambonie et al3 reported the efficacy of nasal-prong CPAP in unloading respiratory muscles and relieving respiratory distress. Martinón-Torres et al16 also found CPAP to be effective in diminishing CO2 values and improving clinical score. These properties seemed to be enhanced by the addition of heliox. Furthermore, other studies (not randomized controlled trials) have shown a decrease in the intubation rate after introducing noninvasive respiratory support (studies cover NIV and CPAP) approaches in infants with severe bronchiolitis.22–24 Proposed reasons for these positive effects of CPAP are diverse. This type of respiratory support has a widening effect on the terminal airways, which prevents airway collapse, recruits underventilated areas, and ultimately improves alveolar ventilation.2,3 This support also seems to reduce airway resistance, which could contribute to a decrease in the work load on the inspiratory muscles. Thus, CPAP is able to reduce the work of breathing and improve ventilation-perfusion mismatch.
Essouri et al25 found that the optimal CPAP level in infants with hypercapnic bronchiolitis was 7 cm H2O. We used a lower CPAP level of about 6 cm H2O, similar to the findings of Milesi et al.4 The need for further respiratory support may seem high in our study (6 of 16 subjects, 37.5%). This might be related to the exclusive inclusion of infants < 3 months old, as young age is one of the most relevant risk factors.26 However, only 2 subjects were eventually intubated, and this intubation rate of 12% was similar to previous studies15,21 or even lower.23,24,26 In any case, the small sample size does not allow further conclusions to be made regarding this point.
Helmet-delivered CPAP has been increasing recently in adult and pediatric populations.7–12,14,27–35 Most studies have shown that the helmet has an increased tolerability and effectiveness,32 with fewer adverse effects than other interfaces.7–9,13,36 However, other researchers have reported better patient–ventilator synchrony with a face mask than with a helmet,28,29,34 although this is of utmost importance when delivering pressure support NIV, and not with CPAP.28,37
In comparison with nasal prongs, the helmet has some theoretical advantages. It allows a good interaction with the environment without limiting an infant's head movements, reducing the risk of dislodging the interface; it avoids fluctuations in the delivered pressure due to oral leaks; and it avoids nasal injuries. During the 2-h study, tolerance was good in both groups (only 3 children required sedatives), and no differences were found. This could be due to the fact that most of these advantages might be more important in the long term, allowing prolonged treatments when using the helmet, as suggested by other authors.7,9 Despite these advantages, it should be taken into account that noise level might be greater when employing a helmet,11 and that the use of a pacifier is challenging, although not impossible.
We used a heated humidifier in all cases. According to some authors, adding this system is advisable for CPAP delivery.38,39 Some experts have described the intermittent use of a heated humidifier to avoid overheating of gases and rain-out effect.9 Heating and humidifying the inspiratory fresh gas flow might help avoid the development of atelectasis secondary to mucous plugging. We also placed a soft cushion inside the helmet for each subject to avoid pressure sores (we had none). Milesi et al11 used a small cushion in the same way after finding pressures sores at the skull base in 3 of the first 6 subjects included in their study.
An important issue that should be addressed when using helmet-delivered CPAP is CO2 rebreathing.11 The volume of the helmet does not directly affect the inspired partial pressure of CO2. The main determinants of inspired partial pressure of CO2 are the amount of CO2 produced by the patient and the flow of fresh gas passing through the helmet.40 In the absence of air leaks, CO2 rebreathing occurs. For this reason, helmet-delivered CPAP should not be performed with closed-circuit (eg, double-limb) ventilators.39 However, some experimental studies did not find significant differences regarding the effective dead space volume among different interfaces (including the helmet).41 We therefore used high gas flows (> 30 L/min) with helmet-delivered CPAP to avoid rebreathing, as other authors do.9
This study has several limitations. The small size of the sample precludes any definitive conclusions. However, it was sufficient to suggest similar clinical response to both methods of CPAP in this special group of subjects. Lack of a washout period between interface changes may have had a carryover effect on the second part of the study. This possible bias was lowered, however, because it was present in both groups. Furthermore, we decided not to establish a washout period because we thought that the clinical situation of a critically ill infant might worsen acutely due to withdrawing CPAP therapy for some time. The non-blinded design of the study is another limitation, which cannot be avoided for obvious reasons. In addition, the use of sedatives was based on caregivers' impressions, not on validated scales. Finally, it would have been very informative to have recorded esophageal pressure, so that we could quantify the relief in the work of breathing with each interface.25,26
This study has some strengths. It is the first randomized study comparing CPAP delivered via helmet and nasal prongs in infants with moderate to severe bronchiolitis. This study focuses on a very specific and challenging population of small infants with bronchiolitis. This type of patient represents one of the more time- and resource-consuming patients during winter periods in most pediatric ICUs, and thus, our study might be of some help for many physicians. In conclusion, our results suggest that CPAP delivered via helmet or via nasal prongs has similar efficacy in very small infants with moderate to severe bronchiolitis.
Acknowledgments
The authors acknowledge the assistance of the medical and nursing staff of our pediatric ICU.
Footnotes
- Correspondence: Juan Mayordomo-Colunga MD PhD, Sección de Cuidados Intensivos Pediátricos, Área de Gestión Clínica de Pediatría, Hospital Universitario Central de Asturias, Avda. de Roma s/n 33011, Oviedo, Asturias, Spain. E-mail: jmcolunga@hotmail.com.
The authors have disclosed no conflict of interest.
- Copyright © 2018 by Daedalus Enterprises