Abstract
BACKGROUND: In patients on mechanical ventilation, lung hyperinflation is often performed to reverse atelectasis and clear retained mucus. We evaluated the effects of manual hyperinflation and ventilator hyperinflation on mucus clearance, gas exchange, pulmonary mechanics, and hemodynamics.
METHODS: Six mechanically ventilated pigs with severe Pseudomonas aeruginosa pneumonia randomly received either 12 manual hyperinflation breaths over a period of 2 min (through a gradual manual compression of a resuscitation bag within 4 s to achieve 40 cm H2O of airway pressure), or 12 ventilator hyperinflation over 2 min to achieve the same ventilatory end points as in manual hyperinflation. Mucus clearance rate was measured through fluoroscopic tracking of tracheal markers. Prior to each maneuver and 15 min thereafter, we assessed arterial and mixed gas exchange, pulmonary mechanics, and hemodynamics.
RESULTS: Both manual hyperinflation and ventilator hyperinflation significantly decreased inspiratory flow by approximately 16 L/min (P < .001) and increased peak expiratory flow by roughly 44 L/min (P < .001). The median (interquartile range) mucus clearance rate was 1.31 (0.84–2.30) prior to the interventions, and 0.70 (0.00–2.58) and 0.65 (0.45–1.47) during manual hyperinflation and ventilator hyperinflation, respectively (P = .09). Hyperinflations, whether delivered manually or through the ventilator, did not significantly modify pulmonary or hemodynamic parameters.
CONCLUSIONS: In an animal model of severe P. aeruginosa pneumonia, neither manual hyperinflation nor ventilator hyperinflation improved mucus clearance. If confirmed in comprehensive clinical experimentations, these findings should promote reappraisal of indications for both manual hyperinflation and ventilator hyperinflation as a therapeutic technique for mucus clearance and atelectasis reversal.
Introduction
In critically ill patients, mechanical ventilation is a life-saving intervention aimed at supporting ventilatory function. Several iatrogenic conditions may complicate the course of mechanical ventilation, especially when patients are deeply sedated and respiratory defenses are consequently impaired. In particular, mechanically ventilated patients who are unable to breathe spontaneously, rapidly develop atelectasis, leading to ventilation perfusion mismatch and impaired gas exchange.1,2 Moreover, following tracheal intubation, the mucociliary escalator is drastically weakened3 and mucus is chronically retained. Indeed, Konrad et al4 demonstrated in ICU subjects a 10-fold decrease in mucociliary clearance rate, as well as higher risks of developing pulmonary infections. Mechanical ventilation has been found to induce a paradoxical displacement of mucus toward the main bronchi in animal models,5–8 which is thought to be driven by gravity and the inspiratory flow.
In operating rooms and ICUs, lung hyperinflation is a technique commonly performed to reverse pulmonary atelectasis and clear retained mucus.9 This could be achieved manually through a resuscitating bag, namely manual hyperinflation,10 or by modifying the ventilatory settings (ie, ventilator hyperinflation).11 During manual hyperinflation, the patient is disconnected from the ventilator and a slow inspiration of a larger tidal volume (VT) is delivered via a resuscitation bag. Then, after an inspiratory pause, the operator releases the inflation bag to ensure a rapid increase in the expiratory flow. Manual hyperinflation presents some inherent risks due to the disconnection from the mechanical ventilator and the marginal control of the amplitude and duration of the lung inflation, which may vary based on the operator's level of training and the loss of PEEP.12 Ventilator hyperinflation is a suitable alternative to avoid such adverse effects.13
To our knowledge, 4 clinical studies have compared the effects of manual hyperinflation and ventilator hyperinflation and found no differences in sputum wet weight, dynamic and static pulmonary compliance, oxygenation, and hemodynamic stability.14 These reports were limited by the use of surrogate outcomes to estimate the effectiveness of the techniques on mucus clearance. Based on these previous results, we hypothesized that manual hyperinflation and ventilator hyperinflation could have comparable benefits through the improvement of mucus clearance. Therefore, we designed this laboratory study in pigs with severe bilateral pneumonia to comprehensively evaluate efficacy and safety of manual hyperinflation and ventilator hyperinflation. In particular, we aimed to compare the effect of manual hyperinflation and ventilator hyperinflation on mucus clearance rate. We also evaluated the effects on pulmonary mechanics, gas exchange, and hemodynamics.
QUICK LOOK
Current knowledge
During invasive mechanical ventilation, lung hyperinflation is one of the most common ventilatory techniques performed to reverse atelectasis and clear retained mucus. Ventilator hyperinflation is a suitable alternative, but studies providing a detailed comparison of the effects of both techniques on mucus clearance are lacking.
What this paper contributes to our knowledge
Our findings in an animal model of severe pneumonia corroborate that neither manual nor ventilator hyperinflation significantly improved mucus clearance. Furthermore, both interventions affected gas exchange and hemodynamic parameters similarly.
Methods
This study was conducted at the Animal Research Laboratories of the University of Barcelona, Spain. Animals were managed according to local Spanish regulations for the care and use of laboratory animals. The protocol was carried out in pigs with severe Pseudomonas aeruginosa pneumonia, enrolled into a concomitant 76-h study to assess new strategies for endotoxin clearance.15
Animal Preparation and Management
Six female Large White-Landrace pigs (32.8 ± 3.1 kg; range, 30–34 kg) were orotracheally intubated with a 7.5-mm inner diameter Hi-Lo endotracheal tube (Mallinckrodt Medical, Athlone, Ireland) and placed on mechanical ventilation (Servo-i, Maquet, Bridgewater, New Jersey) initially set as follows: volume-control mode, VT 10 mL/kg, respiratory rate 15–20 breaths/min, PEEP 3 cm H2O, and FIO2 = 0.4. Ventilatory settings were adjusted throughout the experiment to maintain PaO2 ≥ 95 mm Hg, PaCO2 = 35–45 mm Hg, and plateau airways pressure ≤ 25 cm H2O. A heated humidifier was set to maintain the airway temperature proximal to the Y-piece at 37°C, and the inspiratory line was fully thermo-insulated with foam rubber. During surgical preparation, and throughout the study, anesthesia was maintained with a continuous infusion of midazolam and fentanyl.5 Ultrasound-guided femoral artery cannulation was performed for systemic arterial pressure monitoring and collection of blood samples. We inserted a 7-Fr balloon-tipped Swan-Ganz catheter into the right jugular vein for hemodynamic monitoring and sampling of blood for mixed venous gas exchange. All pigs received continuous intravenous infusion of fluids to maintain fluid balance.
Model of Severe P. Aeruginosa Pneumonia
Following surgical preparation and hemodynamic stabilization, bronchoscopic-guided pulmonary inoculation of 75 mL 108 colony-forming units of pathogenic ceftriaxone-resistant P. aeruginosa was performed, as previously reported.16 After 24 h, pneumonia was confirmed as PaO2/FIO2 dropped to < 100 and the pigs exhibited at least 2 of the following clinical signs: purulent secretions, fever, or leukocytosis.
Randomization
At 24 h after the bacterial challenge, the animals were randomized to receive either manual hyperinflation or ventilator hyperinflation. Then, at 48 h after the bacterial challenge, the other intervention was applied. One hour prior to the procedure, tracheal secretions were aspirated. The animals were positioned prone, with the bed fully horizontal, approximately 15 min prior to the commencement of the protocol and kept in this position for the duration of the interventions. The internal endotracheal tube cuff pressure was set at 40 cm H2O to prevent air leakage during the procedure, and rates of sedatives and analgesics were increased by 20% to ensure absence of spontaneous ventilation and cough reflex.
Manual Hyperinflation.
Pigs were disconnected from the mechanical ventilator, and a self-inflating 1.5-L resuscitation bag (Mark IV Adulto, AMBU, Germany) was connected to the tip of the endotracheal tube. An in-line fresh O2 flow of 15 L/min was delivered. A PEEP valve (Intersurgical, Wokingham, United Kingdom) was connected to the resuscitation bag and adjusted to the level set at the ventilator before disconnection. A gradual two-handed compression of the resuscitation bag was performed every second, until airway pressure reached, within 4 s, 40 cm H2O (Fig. 1A). An in-line pressure manometer was used to monitor the maneuver. This was followed by an inspiratory hold, and a rapid release of the bag to generate an expiratory flow-bias. Twelve hyperinflations (6 breaths/min) were delivered during 2 min, with inspiratory and expiratory time of 4 s and 2 s of inspiratory pause per each breath. Manual hyperinflations were always performed by the same operator (GLB), who has > 10 years of experience in pulmonary management of critically ill patients.
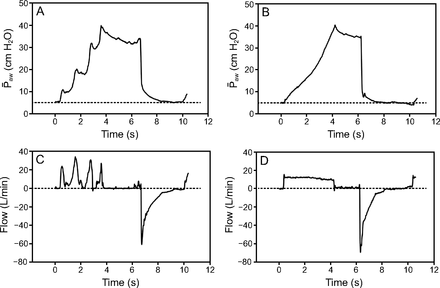
Airway pressures and flows during manual (A, C) and ventilator (B, D) hyperinflations. Upon manual hyperinflation, a gradual compression of the resuscitation bag was performed every second until airway pressure reached 40 cm H2O within 4 s. This was followed by an inspiratory pause of 2 s and a rapid release of the bag to facilitate a high expiratory flow. During ventilatory hyperinflation, tidal volume was augmented until airway pressure reached approximately 40 cm H2O. Inspiratory rise time and inspiratory pause time were set at 0 s and 2 s, respectively, to achieve an inspiratory and expiratory time of 4 s and an inspiratory pause of 2 s. Of note, in both maneuvers, PEEP was maintained accordingly to the previous level on mechanical ventilation. ̄Paw = mean airway pressure.
Ventilator Hyperinflation.
Ventilatory settings were adjusted to achieve 12 lung hyperinflations for 2 min (ie, respiratory rate of 6 breaths/min), as during manual hyperinflation. VT was augmented until airway pressure reached 40 cm H2O, FIO2 increased to 1.0, and the inspiratory-expiratory ratio was set to 1.5:1. Inspiratory rise time and inspiratory pause time were set at 0 s and 2 s, respectively, to achieve an inspiratory and expiratory time of 4 s and an inspiratory pause of 2 s (Fig. 1). PEEP was maintained accordingly to the previous level on mechanical ventilation.
Gas Exchange, Pulmonary Mechanics, and Hemodynamics
Prior to the maneuver and 15 min thereafter, we assessed arterial and mixed gas exchange, pulmonary mechanics, and hemodynamics. As detailed in previous reports,5 we recorded flows, airway pressures, and esophageal pressures; we computed respiratory system, lung, and chest wall elastances, as well as respiratory system, flow, and pulmonary tissue resistances. We also monitored mean arterial pressure, pulmonary artery pressure, central venous pressure, pulmonary artery wedge pressure, and cardiac output. We computed stroke volume, systemic vascular resistance, pulmonary vascular resistance, venous admixture, and oxygen extraction ratio.5
Flows, airway pressures, and esophageal pressures were also continuously recorded during the maneuvers. Thus, we analyzed flow and pressure waveforms of every other breath (6 hyperinflations) and computed the VT, the mean inspiratory flow, the peak expiratory flow (PEF), and the mean expiratory flow from the beginning of expiration to zero-flow. Biases between PEF and mean inspiratory flow and between mean expiratory flow from the beginning of expiration to zero-flow and mean inspiratory flow biases were also calculated as previously described.5 These replicated assessments (6 values) were averaged for the final analysis.
Assessment of Mucus Clearance
Mucus Clearance Rate.
At baseline, we measured tracheal mucus velocity for 10 min via radiographic tracking of radio-opaque disks placed into the trachea and sequential fluoroscopic images taken every 2 min, as previously reported.5 Furthermore, we took 2 fluoroscopic images, one prior to and one upon completion of the interventions, to measure the effects of hyperinflations on tracheal mucus velocity. Only disc movement in the most dependent (ie, ventral) surface were included into the analysis. For each intervention, disk movements were averaged. Mucus rates were characterized by a positive or negative vector when moving toward the glottis or the main bronchi, respectively.
Collected Secretions.
At the end of the maneuvers, tracheal suctioning was performed, and secretions were collected through a 10-Fr suctioning catheter with mucous trap (Argyle Suction Catheter with Mucus Trap, Covidien, Mansfield, Massachusetts). At the end of the suctioning procedure, we squeezed the entire length of the catheter to obtain the remaining mucus adherent to the internal surface. Finally, mucus was aspirated from the mucus trap through a 5-mL syringe. We measured and recorded the wet weight and volume of collected secretions.
Statistical Analysis
Based on the results of our previous studies,5,6 we assumed a typical mean mucus clearance rate of 8 mm/min in animals in the prone horizontal position. In the lung hyperinflation groups, we assumed a 2-fold increase in mucus clearance rate. A standard deviation of 2 mm/min per each group was expected. Thus, we calculated that approximately 6 animals should be included to detect statistically significant differences between the sequential interventions, for a statistical power of 80% and type 1 bias of 5%. Values are reported as mean ± SD or median (interquartile range). Mucus clearance analysis and comparisons of variables collected before, during, and after interventions were performed using a restricted maximum likelihood analysis, based on a repeated measures approach and including the effects of the interventions, times of assessment, and their interactions and day of assessment as additional covariates. A compound symmetry or heterogeneous autoregressive covariance structure was applied. Post hoc comparisons were adjusted using Bonferroni correction. Categorical variables were analyzed using Fisher exact test. All tests were performed 2-sided with a significance level of 5%. Analyses were performed using SAS software (version 9.2; SAS Institute, Cary, North Carolina).
Results
All animals completed the study. At 24 h after bacterial inoculation, pneumonia was confirmed in all animals with the following indications: PaO2/FIO2 decreased by 153.3 ± 73.2 mm Hg (P = .01); purulent respiratory secretions were present in all animals; temperature increased from 36.8 ± 1.1 to 39.0 ± 0.8°C (P = .001); and white blood cells increased from 13.0 ± 3.9 to 17.5 ± 8.0 cells/mm3 (P =.050).
Manual Hyperinflation and Ventilator Hyperinflation Features
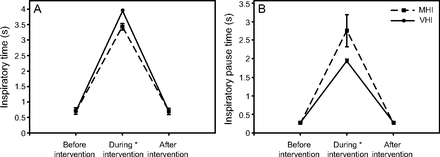
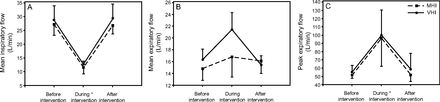
As depicted in Figure 2, interventions prolonged the inspiratory time (P < .001), more so with ventilator hyperinflation (P < .001 vs manual hyperinflation). Similarly, the inspiratory pause time was increased by both interventions (P < .001), more so with manual hyperinflation (P < .001 vs ventilator hyperinflation). VT during manual hyperinflation and ventilator hyperinflation increased from 314.0 ± 24.1 mL to 656.6 ± 150.3 mL and from 327.5 ± 32.2 mL to 838.3 ± 75.1 mL (P < .001), respectively, without significant differences between interventions (P = .18) or days of assessment (P = .32). Delivered VT was highly variable during manual hyperinflation, but it was consistently delivered by ventilator hyperinflation (Fig. 3). Mean ± SD peak inspiratory pressure was not different between interventions (P = .07) or days of assessment (P = .61), but it did differ among times of assessment (P < .001), 24.1 ± 3.1, 38.2 ± 0.6, and 26.9 ± 5.5 cm H2O, respectively, during manual hyperinflation; whereas, during ventilator hyperinflation it was 24.3 ± 3.3, 40.3 ± 1.2, and 24 ± 3.8 cm H2O. Figure 4 details air flows during the interventions; Figure 5 depicts air flow biases. Both manual hyperinflation and ventilator hyperinflation significantly decreased mean inspiratory flow by approximately 16 L/min (P < .001 vs before and after interventions) and increased PEF by roughly 44 L/min (P < .001 vs before and after interventions). As a result, the bias between PEF and mean inspiratory flow increased by 60 L/min during both interventions (P < .001 vs before and after interventions). Mean expiratory flow from the beginning of expiration to zero-flow increased by approximately 1.5 L/min and 5.5 L/min during manual hyperinflation and ventilator hyperinflation, respectively, resulting in substantial increases in the bias between mean expiratory flow from the beginning of expiration to zero-flow and mean inspiratory flow of around 16 L/min and 21 L/min for manual hyperinflation and ventilator hyperinflation, respectively (P < .001 vs before and after interventions).
Inspiratory time and inspiratory pause during ventilator and manual hyperinflations. Data are reported as mean and standard deviation. A: As expected, type of intervention and time of assessment modified the inspiratory time, without differences between days of assessment (P = .48). B: Similarly, for both interventions, times of assessment and the days of assessment (P = .03) modified the inspiratory pause time. *Post-hoc analysis of effect time of assessment (P < .001) vs. before and after treatment. MHI = manual hyperinflation; VHI = ventilator hyperinflation.
VT (mL/kg) delivered during manual hyperinflation (left) and ventilator hyperinflation (right) per each animal. The solid lines depict the average of the 6 assessments; the upper and lower dotted lines indicate the standard error, and dashed lines indicate 95% CI. Recorded data of animal 111 were not available to compute VT delivered manually. On average, VT during manual hyperinflation was 20.2 ± 6.1 mL/kg, whereas VT during ventilator hyperinflation was 25.6 ± 2.7 mL/kg (P < .001). Of note, the hyperinflations manually delivered were highly variable. Conversely, the hyperinflations during ventilator hyperinflation were consistent. MHI = manual hyperinflation; VHI = ventilator hyperinflation; VT = tidal volume.
Flows generated before, during, and after either manual or ventilator hyperinflation. Data are reported as mean ± SD. A: Mean inspiratory flow did not differ between interventions or days of assessment (P =.82) but differed among times of assessment. B: Mean expiratory flow differed among times of assessment. C: Peak expiratory flow did not vary between interventions (P = .63) or days of assessment (P = .95), but they differed among times of assessment (P < .001) * Post-hoc analysis P < .001 vs. before and after treatment. MHI = manual hyperinflation; VHI = ventilator hyperinflation.
Flow biases generated before, during, and after either manual or ventilator hyperinflation. Data are reported as mean ± SD. A: Bias between mean expiratory flow and inspiratory flow did not differ among interventions or days of assessment (P = .63), but there was significant difference among times of assessments. Of note, the manual and ventilator hyperinflations reversed the negative expiratory flow bias. B: The bias between peak expiratory flow and mean inspiratory flow differed among interventions and times of assessment, but without variations between days (P = .97). *Post-hoc analysis P < .001 vs. before and after treatment. MHI = manual hyperinflation; VHI = ventilator hyperinflation.
Effects of Manual Hyperinflation and Ventilator Hyperinflation on Mucus Clearance
A total of 158 movements of discs on the most dependent tracheal regions were averaged and included in the final analysis. Figure 6 depicts the results of mucus clearance studies per intervention and day of assessment. Mucus moved toward the glottis during baseline assessments, and it was decreased by manual hyperinflation and ventilator hyperinflation but did not reach significance (P = .09). Mucus moved toward the main bronchi in 20% and 16.7% of manual hyperinflation and ventilator hyperinflation interventions, respectively, and never during baseline assessments (P = .73). Furthermore, retrieved mucus volume was 0.7 ± 0.4 mL in manual hyperinflation and 0.3 ± 0.2 mL in ventilator hyperinflation (P = .69), which weighed 888.5 ± 563.8 mg in the manual hyperinflation group and 528.8 ± 356.7 mg in the ventilator hyperinflation group (P = .64). Finally, mucus volume was 0.5 ± 0.3 mL during the first day of assessment and 0.6 ± 0.5 mL during the second day (P = .99), and the respective weights were 637.5 ± 344.0 mg and 779.8 ± 626.3 mg (P = .84).
Mucus clearance rate per intervention and day of assessment. Mucus clearance rate did not differ among study intervention (P = .09) and study days (P = .75). At baseline, mucus clearance rate was faster in 3 animals and slower or similar in the 3 remaining animals.
Effects of Manual Hyperinflation and Ventilator Hyperinflation on Gas Exchange, Pulmonary Mechanics, and Hemodynamics
Interventions similarly increased heart rate (P < .001). median (interquartile range) heart rate measurements before, during, and after interventions were 61 (54–73), 72 (62–119), and 73 (67–119) beats/min with manual hyperinflation, respectively; with ventilator hyperinflation, the respective heart rate measurements were 67 (51–79), 80 (69–101), and 83 (64–101) beats/min. Conversely, interventions did not affect mean (range) arterial pressures (P = .54), which were 76.5 (76–87), 83 (78–91), and 87.5 (80–96) mm Hg before, during, and after manual hyperinflation, respectively, and 85.5 (80–86), 90.5 (81–96), and 80 (76–86) mm Hg, respectively, with ventilator hyperinflation. As reported in Table 1, pulmonary mechanics and hemodynamics changed over time, but manual hyperinflation and ventilator hyperinflation affected these parameters analogously.
Pulmonary Mechanics, Gas Exchange, and Hemodynamics
Discussion
The results of this study indicate that manual hyperinflation and ventilator hyperinflation have similar marginal effects on mucus clearance in an animal model with severe P. aeruginosa pneumonia. In addition, both interventions improved expiratory flow bias, and ventilator hyperinflation results in more consistent delivery of lung hyperinflation.
To date, clinical evidence on the effects of manual hyperinflation or ventilator hyperinflation is scant, inconclusive, and lacks objective outcomes to assess the benefits of the interventions. Taking these limitations into account, in several studies manual hyperinflation produced short-term improvements in pulmonary mechanics and surrogate measures of mucus clearance,17 particularly when it was performed with the Mapleson-C circuit18 and combined with the Trendelenburg position.19 Ventilator hyperinflation has been proposed recently as an alternative technique to prevent several of the potential drawbacks of manual hyperinflation, such as ventilatory disconnection and tidal hyperinflation heterogeneity.12 Lemes et al20 evaluated the effects of 30-min ventilator hyperinflation, up to 40 cm H2O, in subjects with pulmonary infection and reported an increased amount of collected mucus. Similar benefits were noted with either technique in a meta-analysis.14 Yet, during ventilator hyperinflation, disconnection from mechanical ventilation is not necessary, and VT and airway pressures are strictly monitored by the ventilator. In this study, ventilator hyperinflation achieved consistent ventilatory end points, as planned for by the protocol. Despite these findings, it is important to highlight that manual hyperinflation remains the most commonly used hyperinflation technique in clinical settings. This could be related to the fact that ventilator hyperinflation is a relatively new method of lung hyperinflation, and health care providers may not be appropriately trained in adjusting the ventilator settings to safely deliver the intervention.
The applied interventions efficiently improved both the bias between PEF and mean inspiratory flow (by 60 L/min) and the bias between mean expiratory flow from the beginning of expiration to zero-flow and mean inspiratory flow (by 16–21 L/min). Nevertheless, in our model of pneumonia the median mucus clearance rate ranged from 0.65 to 1.31. These rates are similar to previous studies in healthy animals5 but do not indicate any beneficial effect of either type of hyperinflation on mucus clearance. It is difficult to explain these negative findings, particularly in view of several prior bench and animal model reports that showed enhanced mucus clearance when the expiratory flow overcame the inspiratory flow.5,8,21 Several features of our settings should be considered to better characterize these findings. First, lung hyperinflation theoretically resembles a cough burst. Efficient cough includes lung hyperinflation, swift glottic closure, and forced expiration.22 Conversely, during both manual hyperinflation and ventilator hyperinflation, the endotracheal tube prevents full closure of the glottis, thus reducing the peak expiratory flow.23 Second, in comparison to standard mechanical ventilation, manual hyperinflation and ventilator hyperinflation increased the inspiratory time 4-fold; thus, the prolonged interaction of the inspiratory air flow with mucus could have slowed the clearance rate rather than promoting it. Third, tracheal suctioning was performed 1 h prior to initiation of the protocol for baseline normalization of retained secretions. Previously, Landa et al24 showed negative effects of suctioning on mucus clearance, but only when prolonged aspiration of up to 24 h was applied. Fourth, our previous studies25 in animals, in a model of semi-recumbent position and on invasive mechanical ventilation, demonstrated that brief and strong rib-cage compressions, synchronized with the early expiratory phase, improved outward clearance of mucus. Thus, if not contraindicated, rib-cage compressions could be applied following hyperinflation as a suitable alternative to enhance airway clearance. Another feasible option, as reported by Berney et al19 and previous laboratory studies,6 would be to position the patient in a slight Trendelenburg position to enhance gravity-driven mucus movement. It should be emphasized that, in our model, animals were kept in a prone position rather than supine like in humans. Indeed, pigs are quadrupeds and commonly develop extensive atelectasis and respiratory derangement when maintained in the supine position for prolonged periods. As previously shown,26 in the prone position the pig trachea is ventrally tilted, which could theoretically favor gravity-driven mucus clearance. In contrast, in the supine position, tracheal orientation both in pigs and humans has an oblique orientation toward the dorsal region, which potentially thwarts mucus clearance. Hence, our negative results should be considered in light of these specific tracheal orientations and potential inter-species dissimilarities. Finally, we measured mucus clearance at the most dependent mid-tracheal regions. In this area, as we previously demonstrated,5,6,27 movement of retained mucus is mainly driven by gravitational force and flows. Thus, during baseline assessments, gravity might have played the most important role on mucus displacement, particularly because animals were positioned fully horizontal.
As for the effects of hyperinflation on gas exchange and pulmonary mechanics, manual hyperinflation improved pulmonary compliance in previous studies,17,28–30 but the effects on arterial oxygenation were inconsistent,17 particularly in subjects with lung injury.31 In addition, manual hyperinflation was often associated with significant decrease in cardiac output,32,33 changes in heart rate,31,34 and, sometimes, an increase in systemic vascular resistance32 and end-tidal carbon dioxide.34 Overall these deleterious effects had limited clinical relevance, specifically in patients with shock or lung injury. In our study, gas exchange and hemodynamics (ie, heart rate and central venous pressure) were altered equally by manual hyperinflation and ventilator hyperinflation. Nevertheless, it should be emphasized that pulmonary and hemodynamic parameters were assessed 15 min after completion of the intervention, potentially lacking temporal association. In particular, because PEEP was not increased following application of the maneuver, the potential gain in pulmonary recruitment could have been lost during the waiting period.
This study has some limitations that should be addressed. First, our laboratory findings should be judiciously extrapolated into clinical scenarios due to the limited number of studied animals and inherent differences between our porcine model (eg, deep sedation and paralysis with absence of cough or any expiratory muscle activity) with the ICU patient. Yet this is the first report to objectively quantify mucus clearance during lung hyperinflation, adding novel insightful information to this field of investigation. Second, animals were kept in a prone horizontal position, rather than in a model of the semi-recumbent position, as in our previous reports.6 Thus, in comparison with patients, who are commonly positioned in the semi-recumbent position, we might have overestimated the effects of hyperinflation on mucus clearance. Moreover, the level of lung hyperinflation might have been thwarted by the abdominal viscera forcing against the diaphragm. Third, we tested the interventions in a model of severe pneumonia and, differently than in clinical settings, we did not increase PEEP after intervention. Thus, the lack of oxygenation improvement should be read in light of the applied methods and may not apply to different models of acute lung injury. Finally, a repeated-intervention study design was applied, and, although we randomized the interventions, potential carryover effect could have occurred.
Conclusions
Our findings reappraise the role of manual hyperinflation and ventilator hyperinflation as techniques to enhance mucus clearance and their utility during mechanical ventilation. Furthermore, in our porcine model of severe pneumonia, manual hyperinflation and ventilator hyperinflation exerted similar effects on gas exchange, pulmonary mechanics, and hemodynamics.
Acknowledgments
We thank the nurses of the Pulmonary Critical Care Unit, Hospital Clinic, Barcelona, for their invaluable and consistent support.
Footnotes
- Correspondence: Antoni Torres MD PhD, Pulmonary and Critical Care Unit Hospital Clínic, Calle Villarroel 170, Esc 6/8 Planta 2 08036 Barcelona, Spain. E-mail: atorres{at}clinic.ub.es.
Drs Li Bassi and Martí are co-first authors.
The authors have reported relationships with Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Ministerio de Ciencia e Innovación (PS09/01249); Fundació Catalana de Pneumologıa (FUCAP); Sociedad Española de Neumología y Cirugía Torácica (SEPAR); Centro de Investigación Biomedica En Red-Enfermedades Respiratorias (CIBERES).
Dr Li Bassi presented a version of this paper at the 26th ESICM Meeting, held October 5-9, 2013, in Paris, France.
See the Related Editorial on Page 870
- Copyright © 2019 by Daedalus Enterprises