Since the ARDSNet low tidal volume study was published in 2000,1 lung-protective ventilation has become the mainstay of conventional ventilatory support for patients with ARDS. However, when clinicians are unable to meet oxygenation or ventilation goals with conventional ventilator support, extracorporeal membrane oxygenation (ECMO) represents a rescue strategy that may have the potential to improve survival.2 ECMO not only allows for a reduction in ventilator support due to augmented gas exchange, it may also markedly reduce the patient’s work of breathing, sometimes resulting in apnea. ECMO facilitates the minimization of ventilator-induced lung injury and enhanced recovery through allowing lung rest. As patients recover, decision-making around when to remove a patient from ECMO to transition back to conventional support is one of the most challenging aspects of ECMO management. Successfully balancing the risks of ECMO versus those of conventional mechanical ventilation are crucial to the patient’s survival.
In this month’s edition of Respiratory Care, Spinelli et al3 present their study demonstrating that the rapid shallow breathing index (RSBI) is correlated with severity of disease in subjects undergoing ECMO with maximum carbon dioxide production removal. In their study RSBI appears to correlate with disease severity in a manner similar to gas exchange by native lung, lung weight, and organ failure. The RSBI has the advantage of being an easily measured bedside test that incorporates breathing frequency and tidal volume directly from the ventilator. The use of RSBI has appeal at the bedside due to its simplicity and rapid calculation. Most other measures of lung disease require imaging or invasive testing. In this study by Spinelli et al,3 an RSBI > 105 was not associated with a statistically significant change in ventilator-free days, duration of mechanical ventilation, or ECMO duration. However, even though this investigation is small and underpowered, there does appear to be trend toward fewer ventilator-free days and longer duration of ventilation and ECMO with a high RSBI. While definitive conclusions cannot be drawn on the basis of this investigation, the analyses do demonstrate a stepwise escalation in severity of disease progressing from patients with apnea to those with normal respirations to those with rapid shallow breathing. Although not statistically significant as an indicator for spontaneous respiratory failure during ECMO with maximum CO2 removal, this parameter does offer some hope as a potential screening tool for patient readiness to wean from ECMO. RSBI can also be easily trended, and in future research it would be beneficial to study the trend of RSBI over time as a potential predictor of the optimal time to transition from ECMO to conventional ventilation.
Another key message that can be inferred from the current investigation is that ARDS is an extremely heterogeneous process, and traditional measures of lung disease severity may not tell the whole story. In this study by Spinelli et al,3 the RSBI index was not correlated with poor compliance or high alveolar dead space, despite its possible correlation with other markers of lung disease severity. These findings certainly may be due to the small number of subjects in the study, but it is possible that the many different phenotypes of ARDS contributed to the findings, and further investigation is warranted to better understand the applicability and prognostic value of RSBI as an important predictive metric. Examination of correlations over time between RSBI and lung weight, compliance, alveolar dead space, biomarkers of systemic inflammation, or other parameters may provide illumination to help providers at the bedside understand new aspects of ARDS pathophysiology, and may even lead to novel prognostic or therapeutic targets.
Furthermore, the variability of RSBI in these subjects with ARDS whose oxygenation and ventilation were augmented and controlled through ECMO informs us that respiratory drive in patients with ARDS is not mediated by 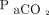 and
and 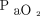 alone. There appear to be other factors in the pathophysiology of severe lung disease that result in tachypnea despite the fact that
alone. There appear to be other factors in the pathophysiology of severe lung disease that result in tachypnea despite the fact that 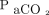 and
and 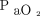 are normalized with ECMO. Processes such as pendulluft, a hyperactive immune response, and increased lung perfusion potentially leading to pulmonary edema can all play a role in the severity of ARDS.4-5 Unfortunately, at this time, we are unable to isolate any single factor that drives work of breathing, and clearly much more work is needed in this area.
are normalized with ECMO. Processes such as pendulluft, a hyperactive immune response, and increased lung perfusion potentially leading to pulmonary edema can all play a role in the severity of ARDS.4-5 Unfortunately, at this time, we are unable to isolate any single factor that drives work of breathing, and clearly much more work is needed in this area.
Publications of this nature are essential steps in advancing knowledge of disease pathophysiology and treatment regimens. However, these works present an important challenge because the reader is left with more questions than answers. As the use of ECMO continues to rapidly expand, works such as these are vitally important to help guide clinicians in attempting to determine the safe and optimal management of this technology. The authors should be commended for their work to further elucidate the impact of ECMO on respiratory drive and effort, and we encourage others to systematically evaluate the potential applications of the RSBI in patients on ECMO.
Footnotes
- Correspondence: John D Davies MA RRT FAARC, Duke University School of Medicine, Box 3911, Duke Hospital, Durham, NC 27710. E-mail: john.davies{at}duke.edu
SEE THE ORIGINAL STUDY ON PAGE 911
The authors have disclosed no conflicts of interest.
- Copyright © 2020 by Daedalus Enterprises







