With the rapid escalation of the COVID-19 pandemic in early 2020 leading to a surge in patients with hypoxemic respiratory failure, several models forecasted an insufficient number of ventilators to support all potentially rescuable patients. In response, hospitals converted operating rooms to ICUs to facilitate using anesthesia machines as ventilators and repurposed noninvasive bi-level ventilators for invasive ventilation. In the United States, ventilators from the national strategic stockpile were deployed. Despite these efforts, concerns remained that available ventilators would be depleted. This raised interest in ventilating more than 1 patient using a single ventilator, inspiring a number of untested videos on social media promoting ventilator sharing. The growing interest led to a joint statement from several American professional societies advising against the use of this approach.1 This could be interpreted to mean that some patients would be triaged to not receive mechanical ventilation. With several hospitals reporting survival of the majority of mechanically ventilated patients with SARS-CoV-2,2-4 some might question why patients should be triaged for mechanical ventilation if a potential alternative is available.
In a bench study, Neyman and Irvin5 split the ventilator output to 4 test lungs of similar compliance and concluded that it was feasible to use a single ventilator for 4 patients. The greatest limitation of this study was the similar compliance of the test lungs and endotracheal tubes with the same resistance; an unlikely scenario in the real world setting of a pandemic. Additionally, measurements consisted of observing the visual rise and fall of the rubber test lungs; there were no measurements of pressure or volume. Paladino et al6 tested this approach on 4 adult human–sized sheep with normal lungs. They reported marked decreases in 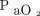 and acute hypercapnia in several animals, likely associated with de-recruitment caused by changes in respiratory mechanics, leading to tidal volume maldistribution between the 4 animals that required intervention. Despite these findings, the authors concluded that it was possible to adequately ventilate the sheep using this system for up to 12 h. Branson et al7 highlighted the limitation of using a single ventilator for 4 patients by altering the respiratory mechanics in each compartment of a lung model, illustrating differences in tidal volume delivery dependent on the compliance and resistance of each compartment.
and acute hypercapnia in several animals, likely associated with de-recruitment caused by changes in respiratory mechanics, leading to tidal volume maldistribution between the 4 animals that required intervention. Despite these findings, the authors concluded that it was possible to adequately ventilate the sheep using this system for up to 12 h. Branson et al7 highlighted the limitation of using a single ventilator for 4 patients by altering the respiratory mechanics in each compartment of a lung model, illustrating differences in tidal volume delivery dependent on the compliance and resistance of each compartment.
The COVID-19 pandemic sparked new interest in ventilator sharing. In a lung model, Tonetti et al8 assessed the potential for ventilating 2 patients with a single ventilator. They correctly recognized the issue of different respiratory mechanics affecting tidal volume distribution between the 2 simulated lungs in the model, and thus introduced the concept of patient matching for this technique to be useful. Epstein et al,9 using a lung model, demonstrated that an abrupt change in respiratory mechanics to one simulated patient (ie, endotracheal tube occlusion) results in high volume and pressure delivered to the other simulated patient during volume control ventilation. Clarke et al10 used a flow restrictor to modify the resistance to a lung model simulating ventilator sharing to 2 lungs of differing compliance. This allowed them to successfully deliver similar ventilation to each simulated lung during volume control or pressure control ventilation. Srinivasan et al11 developed a system for individualized shared ventilation using flow control valves, one-way valves, PEEP valves, and filters; they were able to successfully ventilate 2 pigs with shared ventilation using this system. Han et al12 revived an old concept of a bag-in-the-box to individualize tidal volume delivery to 2 patients from a single ventilator.
Through these studies and anticipated clinical applications, it has become clear that to successfully support multiple patients with a single shared ventilator requires either the patients to have similar respiratory mechanics or the ventilator circuit to be altered to distribute volume and pressure differently to each patient. Particularly because each patient’s course will evolve uniquely, safely supporting more than 2 patients with a shared ventilator would seem extremely challenging and perhaps prohibitively unsafe. In this issue of Respiratory Care, 2 papers13,14 assess the concept of ventilator sharing and suggest potential improvements by modifications to the circuit.
Chatburn et al13 conducted a simulation-based study for ventilating 2 patients with one ventilator. The effects of differences in mechanics (ie, resistance and compliance), volume control versus pressure control, and the placement of one-way valves in the expiratory limb were assessed. As previously described, the authors confirmed the potential for markedly different ventilation for patients with different respiratory system mechanics. They also reported, not surprisingly, different end-expiratory lung volumes for the same level of PEEP when lung mechanics differed. They found that the addition of one-way valves had minor effects on volume distribution in volume control, but not in pressure control. The authors suggested that pressure control ventilation is preferable when a single ventilator is used for 2 patients.
Herrmann et al14 used one-way valves, PEEP valves, and components produced on a 3D printer to individualize the ventilator output to 3 lung models. For volume control ventilation, resistors and one-way valves were placed into the inspiratory limb. For pressure control ventilation, spring-loaded pressure relief valves were placed into the inspiratory limb. In this way, flow was partitioned between the lung models to deliver similar volumes (volume control). Different levels of pressure were set for each lung model to deliver similar volumes (pressure control). PEEP valves were placed into the expiratory limb to allow different PEEP levels to each simulated lung. The authors suggest that volume control, even with inspiratory resistive compensation, should be used as a last resort because changes in the mechanics of one patient could be catastrophic for all connected patients. On the other hand, individualization during pressure control may be possible with inspiratory pressure relief valves, with minimal concern for mechanical interactions or pendelluft, and without the necessity for volume monitoring.
Each of the previous studies share important limitations. A single ventilator brand was used, and thus it is not possible to know if the findings are generalizable to other ventilator brands. This is particularly important when resistors, one-way valves, and 3D-printed components are used, which could affect the performance of some ventilators. A limited number of ventilator settings were simulated, raising the question of generalizability to other settings. None of these approaches was tested on human subjects. Finally, and perhaps most important, is to recognize that these approaches are off-label uses of mechanical ventilation. All ventilators are intended for use with a single patient.
The only human experience with ventilator sharing for acute respiratory failure reported in the medical literature comes from Beitler et al.15 The ventilator was configured with 2 parallel circuits using T-adaptors and antimicrobial filters. No one-way valves, resistors, or pressure relief valves were added to the circuit. Compatible patient pairs were identified primarily on the basis of similar driving pressure, PEEP requirement, and the same respiratory pathogen.16 Tidal volume and pressure were monitored continuously for each patient, and blood gases were measured frequently. A total of 6 subjects (3 pairs) with SARS-CoV-2–associated ARDS underwent ventilator sharing, each for approximately 48 h. All subjects tolerated ventilator sharing without adverse events. Median tidal volume was 5.7 mL/kg predicted body weight during ventilator sharing, and median pH was 7.33. The authors concluded that carefully implemented ventilator sharing can fully support compatible patient pairs with SARS-Cov-2 for at least 2 d.
A few observations can be made regarding the approaches to ventilator sharing. First, many introduce complexity. Although in-line resistors and one-way valves have the potential to better match tidal volume delivery to patients with differing respiratory mechanics, they also have the potential for catastrophic unintended consequences such as incorrect placement of valves, incorrectly set resistors, or failure of either of these components. With complex external modification of circuit air flow or pressure, risk of human error is increased, particularly if shortages in expert personnel accompany ventilator scarcity, as would likely occur during surges in case volume. Second, monitoring airway pressure and tidal volume delivery separately for each patient receiving shared ventilation is mandatory to ensure the safety of both patients. Alarms must be set appropriately, ideally with independent alarms for each patient. Third, pressure control seems safer than volume control for ventilator sharing. For example, pressure control prevents the entire volume output from the ventilator going to one patient if the other patient’s endotracheal tube becomes obstructed. Pressure control and the same PEEP for both patients also limits the risk for pendelluft, thus obviating the need for valves. Still, both patients should be passively ventilated to ensure neither triggers the ventilator and to further prevent gas flow between patients. Fourth, if ventilator sharing is ever used, it should be limited to no more than 2 patients for safety. Moreover, ventilator sharing does not double the number of patients who can be ventilated. Ventilators must be available if patients need to be uncoupled for deterioration or weaning of one of the patients. Finally, ventilator sharing should be limited to the shortest time possible, until ventilators become available either through attrition (eg, extubation or death) or can be delivered from another site.
Ventilator sharing is, at best, imperfect. Even with the use of resistors and valves, or closely matching patients of similar respiratory mechanics, differences in ventilation and gas exchange are to be expected between the 2 patients. How much difference is acceptable? The answer to this question is debated among authorities. We have been trained to precisely set tidal volume, PEEP, and 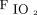 . The range of tidal volume for lung-protective ventilation is 4–8 mL/kg predicted body weight.17 This suggests that the tidal volume of one patient might be twice that of the other and remain within the limits of lung-protective ventilation. If one patient is set for a PEEP of 10 cm H2O and the other for a PEEP of 14 cm H2O, is it okay for both to share a PEEP of 12 cm H2O? Available evidence does not support that precise setting of PEEP affects outcomes.18 Does it matter if the shared
. The range of tidal volume for lung-protective ventilation is 4–8 mL/kg predicted body weight.17 This suggests that the tidal volume of one patient might be twice that of the other and remain within the limits of lung-protective ventilation. If one patient is set for a PEEP of 10 cm H2O and the other for a PEEP of 14 cm H2O, is it okay for both to share a PEEP of 12 cm H2O? Available evidence does not support that precise setting of PEEP affects outcomes.18 Does it matter if the shared 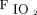 results in an
results in an 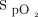 of 90% in one patient and an
of 90% in one patient and an 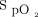 of 94% in the other? In the ARDSnet study, the target
of 94% in the other? In the ARDSnet study, the target 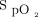 was 88–95%.17 The clinically acceptable ranges in values of respiratory physiological parameters for single-patient ventilation should be maintained during ventilator sharing to ensure both patients are appropriately supported. When this cannot be maintained, a shared ventilator approach is not appropriate.
was 88–95%.17 The clinically acceptable ranges in values of respiratory physiological parameters for single-patient ventilation should be maintained during ventilator sharing to ensure both patients are appropriately supported. When this cannot be maintained, a shared ventilator approach is not appropriate.
There are some important clinical considerations regarding ventilator sharing. It assumes that patients can be matched successfully for similarities in respiratory mechanics and disease trajectory, which might be unrealistic. Neuromuscular blockade is required, which is a potential concern, but this has not been shown to contribute to long-term morbidity if used for up to 2 d.19,20 Its safety profile beyond this duration is unclear, but prolonged neuromuscular blockade likely contributes to neuromuscular weakness and may delay ventilator liberation. Filters must be inserted into the circuit to isolate patients’ respiratory microbes. Humidification during shared ventilation is best provided by a heat-and-moisture exchanger, and ideally a heat-and-moisture-exchange filter is used. However, use of a heat-and-moisture exchanger or heat-and-moisture exchange filter can increase the risk of airway occlusion. Prone positioning may be useful for SARS-CoV-2,2 but it might be challenging with ventilator sharing.
Any approach to ventilator sharing must consider that a patient’s condition is dynamic over time. Perfect patient matching at the onset of ventilator sharing does not prevent one patient from improving or worsening on a different trajectory than the other. In the case of resistors and PEEP valves in the ventilator circuit, even if the result is precise individual patient ventilation at initiation, adjustments are necessary if the patient’s condition changes. Close patient monitoring during ventilator sharing is necessary, as much or more so than is required without ventilator sharing. Despite innovative approaches to alter the circuit, it must be appreciated that this is more than just an issue of plumbing in the ventilator circuit. To emphasize this point, we prefer the term “ventilator sharing,” which implies a shared resource, to “ventilator splitting”, which describes the plumbing and has the connotation of rationing with insufficient support, such as with pill splitting.
Ventilator sharing is a case of the good, the bad, and the ugly. The good is that a patient’s life might be saved when the alternative is certain death. The bad is the complexity of fully supporting 2 patients with a shared ventilator, alterations to the circuit that increase the risk of errors, and the need for closer patient monitoring. Some ventilators must be available if patients need to be uncoupled urgently. The ugly is that errors in ventilator sharing might harm one or both patients if not implemented carefully.
Should we ever consider ventilator sharing to allow 2 patients to share a single ventilator? Perhaps if the only alternative is certain death for a potentially rescuable patient, and if the risk of harm to either patient can be minimized. In a disaster setting in which available ventilators are fewer than the need, clinicians will be faced with the choice to either triage some patients to death or attempt to save 2 patients with one ventilator, which could harm one (or both) of them. These are ethical questions that need to be addressed in consultation with local ethics boards and with administrative oversight. With the increased availably of ventilators during the COVID-19 pandemic, we hope that ventilator sharing will never need to be considered for clinical use. Ventilator sharing is no panacea and should never be approached lightly. Its use requires adequate training of all involved, which is difficult in the setting of a pandemic. It should only be attempted by personnel well versed in respiratory physiology and expert in complex ventilator management. To date, ventilator sharing has not been implemented on a wide scale. We hope that this need will never be realized.
Footnotes
- Correspondence: Dean R Hess PhD RRT FAARC. E-mail: dhess{at}AARC.ORG
Dr Hess has disclosed relationships with Ventec Life Systems, Daedalus Enterprises, Jones and Barlett, McGraw-Hill, and UpToDate. Mr Kallet has disclosed a relationship with Nihon Kohden. Dr Beitler has disclosed relationships with Hamilton Medical and Sedana Medical; he is also supported by grants from the National Institutes of Health (K23HL133489, R21HL145506).
- Copyright © 2020 by Daedalus Enterprises







