Abstract
BACKGROUND: Because impulse oscillometry (IOS) can detect changes in the small airways and is safer to perform during the COVID-19 pandemic than other pulmonary function tests, it may have value in investigating pulmonary sequelae in COVID-19 survivors. This study evaluated the performance of IOS in detecting lung abnormalities in COVID-19 survivors and investigated the associations of the findings with those of lung ultrasound (LUS) and spirometry.
METHODS: In this cross-sectional study, 117 subjects underwent IOS at a frequency range of 4–20 Hz 2 months after COVID-19 diagnosis. They also underwent spirometry and LUS, and their aeration scores were calculated.
RESULTS: On IOS, the resonance frequency was > 12 Hz, and the area under the reactance curve was > 3.60 cm H2O/L/s in 70 (59.8%) and 55 (47.0%) subjects, respectively. A heterogeneity of resistance between R4 and R20 (R4-R20) > 20% was observed in 60 (51.3%) participants. Based on their abnormalities in resistive and reactive parameters, 76 (65.0%) participants had abnormal IOS. Spirometry abnormalities were detected in 40 (34.2%) cases. LUS was abnormal in 51 (43.6%) participants, and the median aeration score was 0 (0–8) points. Abnormal IOS was associated with abnormal LUS (P < .001) and abnormal spirometry (P = .002). Abnormal spirometry had a significant but weaker association with abnormal LUS (P = .031). In participants who reported hospitalization, abnormal IOS was associated with both abnormal LUS (P = .001) and abnormal spirometry (P = .006). In participants who did not report hospitalization, abnormal IOS was associated with abnormal LUS (P < .001) but not abnormal spirometry (P = .063).
CONCLUSIONS: In COVID-19 survivors, IOS detected changes even when spirometry was normal. In these individuals, IOS parameters were more strongly associated with abnormalities on LUS than with abnormalities on spirometry.
Introduction
The burden of caring for patients who survive coronavirus disease 2019 (COVID-19) is likely to be enormous in the coming months and years. Although preliminary data suggest that many survivors of COVID-19 have persistent respiratory damage months after the disease, the extent of long-term respiratory complications remains to be determined.1 The respiratory system is the most affected by COVID-19, with pathological changes including alveolar epithelium destruction, pulmonary vasculature damage, alveolar septal fibrous proliferation, and pulmonary consolidation.2 These changes may result in significant residual lung damage even after the acute phase of pulmonary involvement with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), such as interstitial lung disease and pulmonary hypertension, thus causing damage to pulmonary function for possibly months or even years.2,3
Pulmonary function monitoring is desirable in survivors of COVID-19 with pulmonary involvement during the acute phase of the disease. Although traditional pulmonary function tests (PFTs) (including spirometry, body plethysmography, and the pulmonary diffusing capacity test) are widely available, concern remains regarding the use of these techniques because of the risk of transmission of the virus due to the potential for aerosol formation and coughing during the test.4,5 In this context, a simple and fast method to evaluate respiratory mechanics is impulse oscillometry (IOS), where the need for an individual’s cooperation is minimal since the test is performed during spontaneous breathing.4 IOS involves applying periodic pressure impulses to measure changes in respiratory system impedance (Zrs) that overlap with small-amplitude oscillation waves during spontaneous breathing.6
Alongside PFTs, diagnostic imaging has emerged as a key component of the evaluation of COVID-19 survivors. During the pandemic, lung ultrasound (LUS) has gained prominence in the assessment of these patients because it is dynamic and inexpensive, does not expose the individual to radiation, and minimizes the risk of contamination.7 Because COVID-19 preferentially affects the peripheral areas of the lungs, which are sufficiently accessible to LUS, this method has become an increasingly important tool in COVID-19 clinical practice.7 Although few studies have been performed on the follow-up of this population, LUS signs show high sensitivity and diagnostic accuracy comparable to those of chest computed tomography.8
Because it is a noninvasive and safer technique than traditional PFTs, IOS may have considerable value in the COVID-19 pandemic for investigating the pulmonary sequelae of survivors. IOS is highly successful at detecting peripheral airway disease, an abnormality often associated with viral infections.9 Thus, our objective was primarily to investigate the role of IOS in the pulmonary evaluation of survivors of COVID-19 and secondarily to evaluate the associations of its findings with those of spirometry and LUS in this population.
QUICK LOOK
Current Knowledge
Despite the increasing number of recovered cases, concern regarding the detection of COVID-19 pulmonary sequelae is increasing. Impulse oscillometry (IOS) is tool for evaluating the mechanical properties of the respiratory system because it requires less cooperation from the patient and does not require vigorous respiratory efforts. Among imaging methods, lung ultrasound (LUS) has gained popularity during the COVID-19 pandemic because it is a fast, noninvasive, portable technology that does not use radiation.
What This Paper Contributes to Our Knowledge
Our results showed that 2 months after COVID-19 diagnosis approximately two-thirds of subjects had abnormalities on IOS (including abnormalities compatible with small airway disease), and almost half had abnormalities on LUS. These changes were observed even in subjects with spirometric tests without abnormalities. Importantly, a relationship was identified between IOS parameters and LUS pathological signs. Thus, both IOS and LUS should be considered in the monitoring of COVID-19 survivors with persistent respiratory symptoms.
Methods
Subjects
This was a cross-sectional study conducted in 117 subjects (of 138 eligible patients) treated at Piquet Carneiro Policlìnica, State University of Rio de Janeiro, Rio de Janeiro, Brazil, between August 27, 2020, and February 4, 2021. Subjects age ≥ 18 y with a previous diagnosis of COVID-19 confirmed by reverse transcription-polymerase chain reaction (RT-PCR) within 2 months prior were included. Patients who were still RT-PCR positive at the time of inclusion in the study, had a history of pulmonary resection, neurological/musculoskeletal disease or mental illness, and/or were unable to perform the maneuvers needed for the PFTs were excluded.
The project was approved by the National Research Ethics Committee of Brazil under number CAAE-30135320.0000.5259 and was conducted according to the principles of the Declaration of Helsinki. All participants signed an informed consent form.
Pulmonary Function Testing
IOS was performed using an impulse oscillometer (Quark i2m, COSMED, Rome, Italy). On IOS, lower frequencies (≤ 4 Hz) measure the viscous resistance of the airways, whereas higher frequencies (≥ 20 Hz) reflect the characteristics of the proximal airways.6,10 As no consensus has been established regarding the best resistive parameters to interpret Zr curves, we used the following respiratory system resistance (Rrs) parameters: 4 Hz (R4), 6 Hz (R6), 10 Hz (R10), 20 Hz (R20), the mean resistance between 4 and 20 Hz (Rm), and resistance heterogeneity between R4 and R20 (R4-R20).11 We also evaluated the area under the reactance curve (AX) and the resonance frequency (Fres).9 To reduce aerosol generation, the examination was performed in an environment with HEPA filters, adequate ventilation, hand hygiene, full donning of personal protective equipment, and adequate distance between participants. The laboratory technicians used personal protective equipment, including N95 respirators, goggles, gloves, and aprons. In addition, each participant used disposable filters for viruses and bacteria during the test. The filter resistance was subtracted from the patient’s impedance to correct the error introduced in the measurements by using the device.12 During the IOS evaluation, the participants were instructed to remain seated, maintain their head in a neutral position with manual support of the cheeks and the nostrils occluded by a clip, and then to breathe normally for 40 s.9 The minimum acceptable coherence values were ≥ 0.9 Hz.13
After a rest period of approximately 5 m after completion of the IOS exam, the participants underwent spirometry in a computerized system (nSpire Health, Longmont, Colorado) according to the standards recommended by the American Thoracic Society/European Respiratory Society14 and respecting the calibration guidelines provided by the manufacturer. The predicted values of forced vital capacity (FVC), FEV1, and forced expiratory flow at 25–75% of the FVC (FEF25-75%) were calculated according to the equations of Pereira et al,15 and the results are expressed as percentages of the predicted values. Obstructive disorder was defined by an FEV1/FVC < 0.70, whereas restrictive disorder was inferred from an FVC < 80% of the predicted value in the absence of reduced expiratory flow.16
Lung Ultrasound
LUS examinations were performed on the same day as the PFTs with an Aplio XG device (Canon Medical Systems, Otawara, Japan) with a 3.5–5 MHz convex transducer (working in B mode) or a 7.5–10 MHz multifrequency linear transducer. The convex transducer was routinely used for analysis, whereas the linear transducer was used only when doubts remained regarding the analysis of the pleural surface. After previous training to standardize its performance and interpretation, a team of 6 pulmonologists with experience with the method performed LUS exams (3 with 13 y of experience, 2 with 11 y of experience, and 1 with 8 y of experience with ultrasound at the screening site). Each exam was performed by 2 pulmonologists who reached a consensus in cases of disagreement. Following a 12-zone protocol17,18 and with the participants in a sitting position, LUS exams were performed in 6 areas of each hemithorax (2 anterior, 2 lateral, and 2 posterior). The LUS images were examined to evaluate the following signals: B-lines > 2, coalescent B-lines, and subpleural consolidations. To classify lung injury by LUS, in each of these 6 areas, weights ranging from 1–3 were assigned to each LUS finding as follows: 1 = B-lines > 2, 2 = coalescent B-lines, and 3 = subpleural consolidations. The sum of all 6 areas equaled the aeration score,19 which could range from 0–18 points. After the examination, all ultrasound equipment was sterilized in an isolation room specifically designated for this purpose.
Statistical Analysis
The descriptive analysis consisted of measures of central tendency and dispersion for numerical data and of frequency and percentage for categorical data. The inferential analysis consisted of the Spearman correlation coefficient to measure the associations between the IOS, LUS, and spirometry test measurements and the chi-square or Fisher exact test to compare the categorizations (normal and abnormal) between IOS, LUS, and spirometry. The comparison between subjects who required and did not require hospitalization was performed using Student t test for independent samples or the Mann-Whitney test (numerical data) and the chi-square or Fisher exact test (categorical data). Nonparametric methods were applied because the IOS, LUS, and spirometry measurements did not follow a normal (Gaussian) distribution, as shown by the rejection of the normality hypothesis by the Shapiro-Wilk test. We considered a correlation coefficient ≤ 0.29 weak, between 0.30 and 0.49 moderate, and ≥ 0.50 strong.20 Statistical analysis was performed with SAS 6.11 (SAS Institute, Cary, North Carolina).
Results
Among the 138 patients who were eligible for the study, 21 were excluded for the following reasons: difficulty performing spirometry (n = 10), a history of musculoskeletal disease (n = 4), a history of neurological disease (n = 3), RT-PCR positivity before the protocol (n = 3), and a history of pulmonary resection (n = 1). The final sample consisted of 73 (62.4%) women and 44 (37.6%) men, with a mean age of 54.3 ± 12.6 y. Fifty-four (46.2%) participants reported hospitalization at the time of active disease, with a median of 13 d (7–23) hospitalization. The time from symptom onset to the exams (PFTs and LUS) was 63 ± 10 d. At the time of the exams, 65 (55.5%) and 44 (37.6%) participants had ≥ 1 symptom and ≥ 3 symptoms, respectively. The demographic and clinical characteristics of the studied sample are shown in Table 1.
General Characteristics of the Evaluated Sample
Regarding the PFTs, spirometry showed normal, restrictive, and obstructive patterns in 77 (65.8%), 29 (24.8%), and 11 (9.4%) subjects, respectively. On IOS, Fres > 12 Hz and AX > 3.60 cm H2O/L/s were present in 70 (59.8%) and 55 (47.0%) cases, respectively, following the criteria of Berger et al.21 Considering the values predicted by Oostveen et al22 for Rrs, the R4, R6, R10, and R20 were ≥ 150% in 57 (48.7%), 50 (42.7%), 42 (35.9%), and 36 (30.8%) cases, respectively. An R4-R20 > 20.0%23 was observed in 60 (51.3%) participants. Considering the abnormalities in the resistive and reactive parameters, 76 (65.0%) participants had abnormal IOS. The parameters of the PFTs are shown in Table 2.
Results of Pulmonary Function Tests
The LUS exam was abnormal in 51 (43.6%) participants. The numbers of participants with B-lines > 2, coalescent B-lines, and subpleural consolidations were 50 (42.7%), 32 (27.4%), and 22 (18.8%), respectively. The median aeration score in the study population was 0 (0–8).
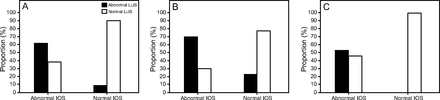
We evaluated associations between the IOS parameters and the LUS and spirometry test findings (Table 3). The LUS aeration score correlated significantly with Fres, Rm, R4, R6, R10, R20, R4-R20, and AX (Fig. 1). Several significant correlations were identified between the IOS parameters and the results of the spirometry test, and the strongest correlation was observed between R4-R20 and FEF25-75%. When we evaluated the associations between LUS signs and spirometry parameters, the LUS aeration score showed significant correlations with the following parameters: FVC (r = −0.44, P < .001), FEV1 (r = −0.41, P < .001), and FEF25-75% (r = −0.34, P < .001). In the total sample, abnormal IOS (R4, R6, R10, or R20 ≥ 150% or R4-R20 > 20%) was associated with both abnormal LUS (P < .001) (Fig. 2A) and abnormal spirometry (P = .002). An abnormal spirometry test was significantly but more weakly associated with abnormal LUS (P = .031).
Relationships between aeration scores and parameters obtained through impulse oscillometry. A: Resonance frequency (Fres, r = 0.42, P < .001); B: the mean resistance between 4 and 20 Hz (Rm, r = 0.53, P < .001); C: resistance at 4 Hz (R4, r = 0.53, P < .001); and D: heterogeneity of the resistance between R4 and R20 (R4-R20, r = 0.58, P < .001).
A: Associations between impulse oscillometry (IOS) parameters and lung ultrasound (LUS) signals in the total sample (P < .001). B: Associations between IOS parameters and LUS signals in participants who reported hospitalization (P = .002). C: Associations between IOS parameters and LUS signals in participants who did not report hospitalization (P < .001).
Correlations of the Impulse Oscillometry Parameters With Lung Ultrasound Signals and Spirometry Test Parameters
Compared to participants who did not require hospitalization (n = 63), participants who required hospitalization (n = 54) had lower values for spirometric indices and higher values for IOS parameters, although without significant differences. On LUS, participants who were hospitalized had higher aeration scores (3 [0–10] points versus 0 [0–2] points, P = .003). The LUS exam was abnormal in 35 (64.8%) and 21 (33.3%) participants who reported and did not report hospitalization, respectively, (P < .001). The correlations of the IOS parameters with LUS signals and spirometric indices were maintained regardless of a previous report of hospitalization. An abnormal spirometric result was observed in 23/54 (42.6%) and 17/63 (27.0%) participants who required and did not require hospitalization, respectively, (P = .053), whereas an abnormal IOS result was observed in 37/54 (68.5%) and 39/63 (61.9%) participants who required and did not require hospitalization, respectively, (P = .45). The LUS exam was abnormal in 30/54 (55.6%) and 21/63 (33.3%) participants who reported and did not report hospitalization, respectively, (P = .02). In participants who reported hospitalization, abnormal IOS was associated with both abnormal LUS (P = .001) (Fig. 2B) and abnormal spirometry (P = .006). In participants who did not report hospitalization, abnormal IOS was associated with abnormal LUS (P < .001) (Fig. 2C) but not abnormal spirometry (P = .063). Abnormal spirometry was not associated with abnormal LUS in participants who reported hospitalization (P = .08) or in participants who did not report hospitalization (P = .16).
Discussion
The main findings of the present study were that whereas only one-third of COVID-19 survivors had an abnormal spirometry test abnormal LUS was observed in almost half of the cases, and abnormal IOS was observed in two-thirds of the cases. The changes detected by IOS were observed in both the resistive and reactive parameters, with abnormalities indicative of peripheral airway disease. In general, the IOS parameters were strongly associated with the LUS signals but only moderately associated with the spirometric indices. Abnormal IOS was associated with abnormal LUS, and this association persisted when the subsamples of participants who required hospitalization and those who did not require hospitalization were analyzed separately. To the best of our knowledge, this is the first study to evaluate the association between 2 relatively safe methods that can be used in the follow-up of survivors of COVID-19: IOS and LUS.
The adverse respiratory outcomes of COVID-19 may be due to direct or immune-mediated attack by SARS-CoV-2 and include pulmonary fibrosis, bronchiectasis, and pulmonary vascular damage, which may persist even after the acute phase of the disease.1,24 Our results showed that only one-third of the survivors had an abnormal spirometry test, with ventilatory damage most often being restrictive and a reduced FVC. FVC findings varied widely: We found an FVC < 80% in 25% of cases, whereas Mo et al2 and Lv et al25 observed a reduced FVC in 9% and 56% of cases, respectively. These discrepancies can be partially explained by the population profile and the time of functional evaluation in the different studies. Lv et al,25 for example, measured lung function 2 weeks after hospital discharge, and most cases were considered severe. Recent evidence shows that up to one-fifth of survivors of COVID-19 develops pulmonary fibrosis, with a consequent decrease in the FVC. SARS-CoV-2 induces its binding to angiotensin-converting enzyme 2 (ACE2), and the risk of developing pulmonary fibrosis is related to higher ACE2 expression in certain risk groups, such as obese and cardiac patients.26
Despite being widely available, spirometry presents problems in the context of the pandemic, including the need for deep exhalation, which increases particle concentrations in the room air, and the requirement of cooperation, which is difficult to achieve in elderly and cognitively deficient people.4 Thus, IOS is a particularly useful test to evaluate the mechanical properties of the respiratory system, and its popularity has been driven by the World Trade Center attack when thousands of workers exposed to smoke and dust showed changes on IOS but not on spirometry.27 In the present study, IOS was more sensitive in detecting abnormalities in lung function, showing such changes in two-thirds of cases. In addition to the survivors of COVID-19 having high mean values for both total (R4) and peripheral resistance (R4-R20), we observed a high mean AX, which can be interpreted as high peripheral resistance or low respiratory system compliance.11 Similar to our study, Huang et al28 used IOS in the post-COVID period in 57 subjects, and subjects with severe disease had higher IOS than those with nonsevere disease. Thus, evaluating respiratory mechanics by IOS can provide important contributions to the monitoring of these patients, especially those with respiratory symptoms.
IOS is a sensitive method for early peripheral airway disease diagnosis in various clinical conditions and can detect peripheral airway disease even before clinical manifestations or spirometric abnormalities appear.5,6,9,22,29 Moreover, the Fres and R4-R20 parameters measured by IOS are considered markers of peripheral airway disease because they signal an increase in peripheral resistance in the respiratory system.11,30 In the present study, a Fres value > 12 Hz and/or an R4-R20 value > 20% was observed in approximately two-thirds of subjects. Because SARS-CoV-2 particles are detectable in the distal epithelial mucosa of the airways by electron microscopy,31 bronchiolitis may arise and lead to peripheral airway disease. These particles may favor a reduction in the caliber of the peripheral airways, which may predispose patients to increased bronchial hyper-responsiveness. Interestingly, we observed a strong correlation between R4-R20 measured by IOS and the FEF25-75% measured by spirometry. In fact, peripheral airway disease seems to be a functional characteristic of survivors of COVID-19, as also found by Mo et al2 and Lv et al25 from terminal flow measurements on spirometry. Although FEF25-75% is viewed by some researchers as an indirect measure of peripheral airway disease, its interpretation should be performed with caution because this parameter strongly depends on expired lung volume.6
The association between pulmonary function and imaging is important in clinical practice because it helps with patient follow-ups. For this reason, interest in the use of LUS as an alternative first-line imaging modality has increased. In COVID-19, LUS can monitor the progression of severe pneumonia after hospital discharge, supporting its integration into clinical models that predict residual lung injury.3 Quantification of LUS findings using scoring systems is effective for monitoring the progression or resolution of lung injury, especially in terms of variations in aeration. In our study, the aeration score was basically due to the presence of > 2 B-lines and coalescent B-lines in almost half of the cases. Whereas B-lines represent thickened subpleural interlobular or intralobular septa, the presence of multiple B-lines per ultrasound field generally corresponds to the ground-glass opacity pattern.7,8,32,33 We observed a moderate correlation between the aeration score and FVC, which may be a consequence of diffuse alveolar damage with varying degrees of early organization and pulmonary fibrosis that occurs in late stages of COVID-19, along with lung volume loss and reduced lung compliance.34,35
We observed several strong correlations between the aeration score and the resistive parameters of the respiratory system measured by IOS. The high rate of functional damage shown by IOS can be explained by the high prevalence of structural lung damage during the acute phase of COVID-19, as previously demonstrated by Carfi et al,36 where 72.7% of individuals developed interstitial pneumonia during hospitalization and 43.4% reported dyspnea approximately 1 month after discharge. Notably, significant correlations were found between both Fres and R4–R20 and the aeration score. The high peripheral resistance may be at least partly explained by the formation of mucosal plugs, bronchial mucosal injury, and closure of the small airways, resulting in a decreased caliber of the more distal airways.24,35 In participants previously hospitalized for COVID-19, we observed higher aeration scores, which may be related to either lung damage by SARS-CoV-2 or pleuropulmonary complications during hospitalization (including bacterial pneumonia, pulmonary embolism, pneumothorax, and pleural effusion).2,8,19,37 Interestingly, abnormal IOS was associated with abnormal LUS even when the subsamples of participants who required hospitalization and those who did not require hospitalization were analyzed separately, suggesting that the more severe pulmonary involvement in hospitalized patients impacts lung structure and function equally.37
Our study has limitations. First, the lack of PFT results before SARS-CoV-2 infection complicated comparisons of our results with the data from the recrudescence phase of the disease. However, since only a minority of subjects had chronic respiratory disease (none of them had severe air flow limitation), we believe that we can reasonably speculate that the pre-COVID pulmonary function of most subjects was normal. Second, since our study was cross-sectional, the long-term dynamic variation in lung function after hospital discharge still requires further investigation. Thus, long-term studies are needed to assess whether the abnormalities on PFTs and LUS persist. Third, we did not use CT in this study, but a recognized limitation of LUS is that it cannot detect deep lung lesions because aerated lungs block ultrasound transmission.8 Fourth, LUS is an operator-dependent imaging modality requiring adequate training to be used reliably by physicians with different backgrounds. We did not evaluate inter-observer and intra-observer reliability, although this point is an open question for LUS38; a recent study showed that intraclass correlation coefficients in the diagnosis of B-lines were good to excellent (range 0.69 to 0.99) and excellent (range 0.88 to 0.90) for inter-observer and intra-observer reliability, respectively.39 Despite these limitations, the clinical relevance of IOS and LUS is evident because they are safe, noninvasive methods that can detect incipient changes in the post-COVID-19 period.
Conclusions
In survivors of SARS-CoV-2 infection, IOS can detect changes even when spirometry is normal. Changes in IOS parameters occur in both the resistive and reactive properties of the respiratory system, and changes consistent with peripheral airway disease are frequent. In these individuals, IOS parameters are more strongly associated with abnormalities on LUS than with abnormalities on spirometry. Thus, the use of IOS and LUS should be considered in the follow-up of patients after acute SARS-CoV-2 infection, especially those whose respiratory symptoms persist. Future studies are needed to investigate the long-term persistence of damage to the respiratory system (either functional or structural) in survivors of COVID-19.
Footnotes
- Correspondence: Agnaldo J Lopes PhD, Rua Araguaia, 1266, bloco 1/405, Freguesia, Jacarepaguá, 22745–271, Rio de Janeiro, Rio de Janeiro, Brazil. E-mail: agnaldolopes.uerj{at}gmail.com
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnólogico (CNPq; grant number #302215/2019-0), Brazil, and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ; grant numbers #E-26/202.679/2018 and #E-26/010.002124/2019), Brazil. Dr Lopes received all these grants from CNPq and FAPERJ.
The authors have no conflicts to disclose.
- Copyright © 2021 by Daedalus Enterprises