Abstract
BACKGROUND: The use of high-frequency oscillatory ventilation (HFOV) is backed by sound physiologic rationale, but clinical data on the elective use of HFOV have been largely disappointing. Nonetheless, HFOV is still occasionally used as a rescue mode in patients with severe hypoxemia. The evidence that supports this practice is sparse.
METHODS: This was a retrospective single-center analysis that involved subjects admitted to the medical ICU at Cleveland Clinic, Cleveland, Ohio. We included all adult patients (ages > 18 y) who received rescue HFOV between January 1, 2010, and December 31, 2018, and analyzed their clinical outcomes.
RESULTS: A total of 48 subjects were included in the analysis. The most common primary diagnosis was pneumonia (n = 33 [68.8%]), followed by aspiration (n = 6 [12.5%]) and diffuse alveolar hemorrhage (n = 2 [4.2%]). Switching to HFOV improved oxygenation but also increased vasopressor requirements at 3 h. The mortality rate of the study population was 92% (44/48).
CONCLUSIONS: Our study did not support utilization of HFOV as a “last-ditch” rescue measure in subjects with respiratory failure. The delayed timing of HFOV initiation and its detrimental hemodynamic effects are among the potential reasons for the high mortality rate.
Introduction
Mortality associated with ARDS remains high.1 The focus of current therapeutic strategies is to sustain life and maintain gas exchange while minimizing ventilator-induced lung injury. For decades, the rationale for the use of high-frequency oscillatory ventilation (HFOV) has been driven by its ability to provide positive-pressure ventilation in a manner that minimizes known causes of ventilator-induced lung injury while providing relatively high mean airway pressures.2 The majority of the clinical investigations on HFOV have explored the role of HFOV in early ARDS. Two large multi-center, randomized controlled trials, OSCAR3 and OSCILLATE,4 were performed to explore the role of early application of HFOV in moderate-to-severe ARDS; although the former trial showed no difference in mortality compared with conventional ventilation, the latter trial was stopped early due to a higher risk of mortality with the use of HFOV. These trials, along with other meta-analyses, quelled any enthusiasm toward the routine early application of this modality in ARDS.5
HFOV has also been used as a rescue therapy for patients in severe respiratory failure in whom conventional mechanical ventilation has reached its limit to provide gas exchange.6 Various reports have highlighted this application and have recommended or even reinforced the persistent consideration of HFOV in the rescue armamentarium.7-9 In a similar vein, a post hoc analysis of HFOV clinical trials by Meade et al10 also showed a trend toward improved mortality with HFOV in subjects with severe ARDS 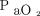 /
/ ≤ 100 mm Hg). Nonetheless, robust clinical evidence that explored the role of HFOV as a rescue therapy in acute respiratory failure is lacking. Specifically, no randomized trials or observational studies have investigated clinical outcomes when HFOV was used as a rescue modality. The objective of this study was to evaluate the clinical outcomes of subjects with acute respiratory failure in whom HFOV was started as a rescue intervention at a major academic medical center.
≤ 100 mm Hg). Nonetheless, robust clinical evidence that explored the role of HFOV as a rescue therapy in acute respiratory failure is lacking. Specifically, no randomized trials or observational studies have investigated clinical outcomes when HFOV was used as a rescue modality. The objective of this study was to evaluate the clinical outcomes of subjects with acute respiratory failure in whom HFOV was started as a rescue intervention at a major academic medical center.
QUICK LOOK
Current knowledge
Utilization of high-frequency oscillatory ventilation as an elective mode in respiratory failure in adults is generally not recommended based on currently available data. However, its role as a rescue mode remains unclear.
What this paper contributes to our knowledge
In our retrospective analysis, the use of high-frequency oscillatory ventilation as a rescue therapy was associated with high mortality and was significantly higher when compared with other rescue therapies. These findings bring into question the utility of high-frequency oscillatory ventilation in this setting.
Methods
This was a retrospective single-center analysis that involved patients admitted to the medical ICU at Cleveland Clinic, Cleveland, Ohio. We included all adult patients (ages > 18 y) who received rescue HFOV between January 1, 2010, and December 31, 2018. Rescue HFOV was defined as the application of HFOV (Sensormedics 3100B, Yorba Linda, CA) when conventional ventilation was unable to provide safe ventilation or gas exchange in the setting of severe hypoxemic respiratory failure. The exclusion criterion was any patient who received HFOV in pediatric or neonatal ICUs. The decision to initiate HFOV was made by the treating intensivist. Respiratory therapists trained on application of HFOV were present 24 h a day and followed an explicit protocol (Appendix 1 [see the supplementary materials at http://www.rcjournal.com]). Adherence to the protocol was not assessed. The study was approved by the institutional review board.
Data were obtained from the electronic medical records. The following data points were extracted and analyzed: baseline characteristics, in-hospital mortality, ventilator settings, hemodynamic parameters, gas exchange data, and use of other rescue therapies. Baseline characteristics included demographics and primary admission diagnosis. We calculated the Charlson comorbidity index,11 the APACHE (Acute Physiology and Chronic Health Evaluation) III score,12 and SOFA (Sequential Organ Failure Assessment) score.13 Reasons to initiate or terminate HFOV were noted. Ventilator and gas exchange data were recorded immediately before and after the initiation of HFOV. Hemodynamic data were recorded at the time of initiation of HFOV and, subsequently, at 3 h, 12 h, and 24 h. Vasopressor doses were converted into norepinephrine equivalent doses to facilitate comparison.14
Statistical analysis was performed by using the SAS 9.4 for Linux (SAS Institute, Cary, North Carolina). The subject information collected was summarized as mean ± SD and median (25th percentile, 75th percentile) for continuous variables, and as counts and percentages for all categorical variables. The study group was divided into 2 groups (alive, dead). The Kruskal-Wallis test was performed to compare non-normally distributed continuous variables. For categorical variables, the Fisher exact test was conducted when one or more of the cells had an expected frequency of ≤5. A linear mixed-effects model was performed to analyze the effect of HFOV initiation on hemodynamics over time (3 h, 12 h, and 24 h). The Wilcoxon signed-rank test was used to compare clinical variables before and after HFOV. The Spearman correlation was performed to analyze associations. The level of statistical significance was set at P < .05 (2-tailed).
Results
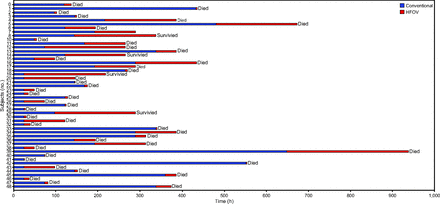
A total of 48 subjects were included in the analysis; their baseline characteristics are presented in Table 1. Of the 48 subjects, 45 (94%) had ARDS and 93% of these (42/45) met the criteria for severe ARDS; the remaining 3 subjects had moderate ARDS with bronchopleural fistula. The most common primary diagnosis was pneumonia (n = 33 [68.8%]), followed by aspiration (n = 6 [12.5%]) and diffuse alveolar hemorrhage (n = 2 [4.2%]). One subject each had the diagnosis of acute interstitial pneumonia, acute lung transplantation rejection, exacerbation of COPD, and extrapulmonary ARDS secondary to septic shock. Other therapies for ARDS were used to a varying extent, both before and after initiation of HFOV. Neuromuscular blockers were administered in 75% (n = 36), inhaled pulmonary vasodilators in 58% (n = 28), and prone positioning in 13% (n = 6) of the subjects. The average time spent on conventional ventilation before initiation of HFOV was 6.83 ± 6.3 d (range, <1 to 27 d). A graphic representation of the time on pre-HFOV conventional ventilation and on HFOV is presented in Figure 1.
Baseline Characteristics of the Subjects at the Time of HFOV Initiation
Graphic representation of the time spent on conventional ventilation and high-frequency oscillatory ventilation (HFOV) in all the subjects.
The ventilator settings before and after switching to HFOV are summarized in Table 2. The most common mode of conventional ventilation was pressure control continuous mandatory ventilation with set-point targeting (70% [n = 34]).15 The remainder were on volume control continuous mandatory ventilation with set-point targeting (23% [n = 11]) or pressure control intermittent mandatory ventilation with set-point targeting for mandatory and spontaneous breaths, specifically, airway pressure-release ventilation (6.4% [n = 3]). The average tidal volume on conventional ventilation was 7.6 mL/kg ideal body weight. The tidal volume delivered was <6 mL/kg ideal body weight in 14.6% of the subjects (7/48) and >8 mL/kg ideal body weight in 31.3% of the subjects (15/48).
Ventilator Settings for Both Conventional and HFOV Modes
Switching from conventional ventilation to HFOV led to a significant increase in mean airway pressure by 8.4 ± 5.2 cm H2O. The mean airway pressure in conventional ventilation was significantly different to the HFOV (23.1 ± 6.7 cm vs 31.5 ± 6.2 cm H2O; P < 0.001). The change to HFOV increased the 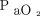 /
/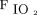 (Δ
(Δ  /
/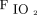 = 13.8 ± 40.6 mm Hg; P = .004) and of the
= 13.8 ± 40.6 mm Hg; P = .004) and of the 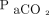 (Δ
(Δ 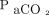 = 9.0 ± 22.1 mm Hg; P = .01) (Table 3). The change in
= 9.0 ± 22.1 mm Hg; P = .01) (Table 3). The change in 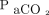 with HFOV showed a positive correlation with the frequency setting of HFOV (P = .039) as well as the pre-HFOV minute ventilation (P = .01), and showed no correlation with the amplitude setting.
with HFOV showed a positive correlation with the frequency setting of HFOV (P = .039) as well as the pre-HFOV minute ventilation (P = .01), and showed no correlation with the amplitude setting.
Gas Exchange Data Before CMV and After Switching to HFOV
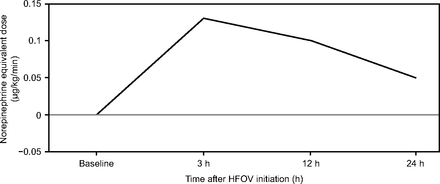
At the time of initiating HFOV, 63% of the subjects were on vasopressors, the average norepinephrine equivalent dose of the group was 0.31 ± 0.41 µg/kg/min. After transition to HFOV, 71% of the subjects were on vasopressors at 3 h, 70% at 12 h, and 67% at 24 h. Transitioning to HFOV showed an increase in vasopressor requirement, which was statistically significant at 3 h but not at 12 h and 24 h. Compared with baseline, the average norepinephrine equivalent dose increased by 0.13 ± 0.44 µg/kg/min (n = 41; P = .002) at 3 h, 0.10 ± 0.41 µg/kg/min (n = 29; P = .19) at 12 h, and 0.05 ± 0.24 µg/kg/min (n = 27; P = .30) at 24 h (Fig. 2). There was also an increase in the average lactate level at 24 h (1.2 ± 2.9; P = .004).
Trends of vasopressor requirements expressed as norepinephrine equivalent dose after initiation of high-frequency oscillatory ventilation (HFOV).
The average time on HFOV was 72.6 ± 62.6 h. The most common reason for termination of HFOV was death, followed by worsened hypercapnia and worsened hemodynamics. Notably, in 2 subjects, HFOV was terminated due to device malfunction. Five subjects were subsequently switched back to conventional ventilation due to improvement in gas exchange. Of the 44 subjects who died, 73% (n = 32) were transitioned to comfort care before their death. 2 of 48 subjects were deemed suitable candidates for venovenous extracorporeal membrane oxygenation. Both of these subjects died, one after 12 d and one after 14 d, from circulatory shock and refractory hypoxemia, respectively. The major cause of death was circulatory shock (54.7% [n = 29]), followed by refractory hypoxemia (35.8% [n = 19]). The mortality rate of the study population was 92% (44/48). Among the 4 subjects who survived, 2 had severe pulmonary ARDS from pneumonia, one had diffuse alveolar hemorrhage, and the fourth subject had moderate ARDS with bronchopleural fistula. All 4 survivors were alive 1 year after hospital discharge.
Discussion
The present study showed that the use of HFOV as a rescue modality was rare and sporadic, averaging ∼6 per year, and was associated with a high mortality rate (92%). We showed that HFOV was generally chosen as a last-ditch effort in patients in extremis. Initiation of HFOV was associated with a higher rate of hemodynamic instability, as witnessed by an increase in vasopressor requirements at 3 h as well as an increase in lactate levels at 24 h. Use of HFOV led to an improvement in oxygenation but worsened hypercapnia and did not change the overall trajectory or course of the disease in these subjects.
The mortality rate noted in our study group was significantly higher than the mortality rate observed in subjects treated with other rescue therapies. For instance, in the ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) trial,16 in which rescue venovenous extracorporeal membrane oxygenation in severe ARDS was studied, the mortality rate was 35% (44/124) in the venovenous extracorporeal membrane oxygenation arm. Similarly, in the Conventional ventilation or ECMO for Severe Adult Respiratory failure (CESAR) trial,17 the subjects allocated to consideration of extracorporeal membrane oxygenation had a 6-month mortality of 37% (33/90). The evaluation of our case series leads to many questions with regard to the way we apply rescue strategies. The high mortality is in contrast to historical reports and experience from other centers, but these results need to be assessed in the context of the use of HFOV as a last ditch effort. The delayed initiation of HFOV in patients with severe disease highlights the tendency to use this modality only after exhausting all avenues with conventional ventilation, a path that may leave patients in more precarious situations than if rescue therapies were triggered earlier. Also, in recent years, HFOV was used in patients who were not considered candidates for extracorporeal life support (ECLS) or as a bridge to goals-of-care discussions. A high number of patients were transitioned to comfort care soon after HFOV initiation.
We also have to evaluate the logistics of the use of an intervention that is rarely used. Even with explicit protocols and annual training, the application of HFOV requires clinical expertise that comes from consistent and repetitive exposure.18 This is the case in neonatology, in which practice has been common and in which clinical outcomes in HFOV have been favorable, especially in patients with hypoxemic respiratory failure.19 Of the average 150–200 cases of severe ARDS admitted to our ICU, only 6 patients/year were started on HFOV. Even though respiratory therapists receive annual training to maintain competency and many of the clinical providers had experience with the device, this limited exposure is perhaps not enough to justify using this modality in a modern ICU. Also, the HFOV runs were short and in extreme circumstances, which limits the exposure and building of experience.
Despite this limited exposure, analysis of the the post-HFOV gas exchange measurements suggests appropriate initial settings and use. There was improvement in the 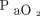 /
/ and an anticipated modest increase in
and an anticipated modest increase in  . The increase in
. The increase in 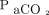 positively correlated with the frequency setting of HFOV, as expected. The Δ
positively correlated with the frequency setting of HFOV, as expected. The Δ  also showed a positive correlation with the pre-HFOV minute ventilation requirement, which can be reflective of a higher physiologic dead space. Notably, despite initial improvement in oxygenation, refractory hypoxemia was the primary cause of death in 35.8% of the cases.
also showed a positive correlation with the pre-HFOV minute ventilation requirement, which can be reflective of a higher physiologic dead space. Notably, despite initial improvement in oxygenation, refractory hypoxemia was the primary cause of death in 35.8% of the cases.
We found that HFOV initiation was associated with early hemodynamic deterioration, as indicated by a significant increase in vasopressor requirements at 3 h. This may have been due to the 36% increase in mean airway pressures on HFOV. Although the rise in the vasopressor dosage > 3 h was blunted, lactate levels at 24 h were found to be higher. Furthermore, circulatory shock was recognized as the most common primary cause of death in our study population. Undesirable hemodynamic effects of HFOV are likely to be more evident in patients who are in extremis. Concerns about the hemodynamic consequences of HFOV have been cited to explain the negative results in the OSCILLATE trial.4 In a study of 16 subjects, use of a higher mean airway pressure with HFOV led to an increase in the incidence of right ventricular failure, as assessed with echocardiography.20
The current results raise serious doubts about the role of HFOV in general. We do believe HFOV, as a mode, has features that can allow adequate ventilation of patients in very specific conditions. The capacity of HFOV to increase mean airway pressures and maintain ventilation with small tidal volumes is unparalleled. It is possible that newer approaches in applying HFOV or protocols that focus on not only gas exchange but also on hemodynamics and respiratory mechanics could potentially still work. However, the current technology is old and lacks safety features and the ability to support spontaneous breathing, the clinician exposure is limited, and the right timelines and clinical scenarios to consider HFOV as a rescue modality are not defined. Furthermore, other rescue strategies, such as prone positioning and ECLS, have become more prevalent.21 All these considerations leave HFOV as a poor choice in a 21st century ICU.
To our knowledge, this was the largest case series of a real-world experience with rescue HFOV. Our study also had several limitations. It was a retrospective study, lacked a control group, and was in a low-exposure center. Several variables (eg, detailed pulmonary mechanics, recruitment maneuvers, and fluid resuscitation) could not be abstracted from the records, which would have given a better picture of the severity of illness. It is possible that the results in higher-use centers with specific protocols might be different or better, to our knowledge, no such report is available. The study population was heterogeneous, and there was no enforcement or evaluation of adherence to the protocol for the utilization of HFOV. Although vasopressor requirement is an important hemodynamic parameter, cardiac output measurement and echocardiographic assessment of the right heart would have detected the hemodynamic effects of HFOV with higher precision.
Conclusions
Our findings bring into question the role of HFOV as a rescue therapy in acute respiratory failure. Further reports from other centers may help define the timing of initiation, patient selection, and management of the hemodynamic consequences. The high mortality rate demonstrated with rescue HFOV makes it a less-desirable option, especially in the current times when the prevalence and feasibility of ECLS is increasing. When conventional mechanical ventilation is unable to provide sufficient gas exchange, early consideration for ECLS or transport to an ECLS center, if not available locally, may be better alternatives to rescue HFOV. If patients are not appropriate candidates for ECLS, then efforts should be focused on optimizing conventional ventilation and addressing goals of care.
Footnotes
- Correspondence: Eduardo Mireles-Cabodevila MD, Department of Critical Care Medicine, Respiratory Institute, Cleveland Clinic, 9500 Euclid Ave, Cleveland, OH 44915. E-mail: mirelee{at}ccf.org
Mr Chatburn discloses relationships with IngMar Medical, Vyaire Medical, Inovytec, and Promedic LLC. The other authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2021 by Daedalus Enterprises