Abstract
BACKGROUND: Recent studies have demonstrated that even in the absence of lung impairment as determined by spirometry, smoking and respiratory symptoms are associated with poor overall health and well-being. However, this relationship is not well defined; and it remains unclear the degree to which symptoms are related to poor health, independent of smoking. This is of particular importance to older adults, as they are more likely to exhibit respiratory symptoms and are, therefore, at risk of not receiving appropriate treatment if they have never smoked and have normal spirometry.
METHODS: We performed a cross-sectional analysis of data from the Canadian Longitudinal Study on Aging to delineate the associations of respiratory symptoms and smoking on the health of participants age 45–86 who exhibited normal spirometry. Participant health was estimated using a frailty index, a multidimensional measure of vulnerability to adverse outcomes that has been validated in numerous health settings.
RESULTS: Of the 21,293 participants included in our analysis, 87% exhibited a normal FEV1, FVC, and FEV1/FVC; of those, 45% reported at least one respiratory symptom, and 50% were former or current smokers. Both respiratory symptoms and smoking were independently associated with frailty (median interquartile range [IQR] = 0.11 [0.07–0.15]), the most substantial associations observed for those having at least one respiratory symptom (adjusted β 0.023, 95% CI 0.022–0.025) and current smokers with > 10 pack-year exposure (adjusted β 0.014, 95% CI [0.010–0.019). Not only was the association between symptoms and frailty evident in never smokers, a significant proportion of the total effect of smoking on frailty was observed to be mediated by symptoms.
CONCLUSIONS: Our data show that respiratory symptoms, regardless of smoking history, were a significant correlate of frailty in older adults with normal spirometry. Hence, they should not be simply regarded as a benign by-product of aging.
Introduction
Spirometry remains the most widely used tool to assess pulmonary function and establish lung impairment.1 This test measures the FEV1 and FVC, abnormalities of which are helpful in making the clinical diagnosis of chronic obstructive and restrictive lung diseases.2 However, recent studies have shown that a substantial proportion of adults with underlying airway disease has normal spirometry. Woodruff and colleagues3 showed that former or current smokers with > 20 pack-years and without spirometric evidence of obstruction (FEV1/FVC < 0.7) utilized more health care services than healthy never smokers, regardless whether they reported respiratory symptoms such as cough, dyspnea, activity limitation, and chest tightness; further, relative to asymptomatic subjects, those reporting symptoms demonstrated greater activity limitations and airway-wall thickening. Similarly, Regan and colleagues4 reported that adults with normal spirometry (FEV1/FVC < 0.7 and predicted FEV1 ≥ 80%) and at least 10 pack-years exhibited worse quality of life, reduced physical function, and evidence of airway thickening or emphysema as compared to never smokers.
Whereas it is clear from this work that a history of smoking and presence of respiratory symptoms are strong indicators of poor health and adverse outcomes, even in those with normal spirometry, the exact relationship between these factors is unclear. This is particularly important for older adults with normal spirometry given that respiratory symptoms are more common5 and, therefore, more likely to be dismissed by primary health care providers. Whereas respiratory symptoms have been previously shown to be a significant predictor of all-cause and cardiopulmonary-specific mortality,6 little else is known regarding the role of symptoms in the context of lung function and smoking history. A valuable correlate to investigate in this regard would be frailty, a multidimensional measure of health that represents one’s vulnerability to adverse outcomes in response to significant stressors such as illness or injury7; examples of frailty-related outcomes include Alzheimer disease and dementia,8 disability and health care utilization,9 and mortality.10 Although smoking is known to be associated with both prevalent11 and incident frailty,12 the impact of respiratory symptoms on frailty is poorly described; and to our knowledge, neither factor has been studied in the context of older adults with normal spirometry.
In the following study, we sought to investigate the associations of smoking and respiratory symptoms with frailty in a large, community-based sample of older Canadian adults with normal spirometry. We hypothesized that not only would respiratory symptoms be prevalent in this sample but that they would be associated with frailty regardless of smoking status.
QUICK LOOK
Current Knowledge
Recently published data indicate that a clean bill of health following spirometric lung function testing does not exclude underlying pathology, which impacts both function and well-being. Even after considering smoking status, an important role for respiratory symptoms is suggested but has not been investigated.
What This Paper Contributes to Our Knowledge
Our analysis of more than 20,000 older adults from a population-based sample found that even in older adult smokers with normal lung function on spirometry symptoms were prevalent and related to frailty. Therefore, these symptoms should not be dismissed as a benign by-product of aging.
Methods
Cohort Description and Participants
This study was a cross-sectional analysis of data from the Canadian Longitudinal Study on Aging (CLSA) baseline collection (2012–2015); the CLSA study design and methods have been previously described.13 It was based on a subgroup of CLSA participants belonging to the comprehensive cohort, which included 30,097 community-dwelling adults age 45–85 who provided questionnaire data through in-home interviews and additional physical and cognitive data at one of 11 data collection sites nationwide. This study and its protocol was approved by the Health Sciences North Research Ethics Board (# 21–009).
In our analyses, we excluded participants if they had a contraindication to lung function testing (n = 3,359) or generated spirometry readings below grade B (n = 5,399; see below). We also excluded all participants missing any spirometry data (n = 1) or exhibited an FEV1/FVC (see below) > 1.0 (n = 8) or in whom smoking status could not be defined (n = 46; see below). The final sample included 21,293 participants.
Lung Function Measures and Definition of Normal Spirometry
Lung function was assessed by a trained technician using a portable spirometer (TrueFlow Easy-on PC spirometer, ndd Medical Technologies, Zürich, Switzerland) without use of bronchodilators. The maximum FEV1 (L/s) and FVC (L) were obtained for each participant and were used to calculate the FEV1/FVC ratio. Participants were allowed up to 8 attempts to provide 3 acceptable spirometry measures, determined by the duration and consistency of the flow-rate pattern of each maneuver. Age, sex, height, and ethnicity standardized values (Z scores) for FEV1, FVC, and FEV1/FVC were derived using equations from the 2012 Global Lung Function Initiative14 and software developed by Theodore Lytras (https://github.com/thlytras/rspiro/). Height (m) was measured by stadiometer, and ethnicity was based on interviewer assessment. Missing ethnicity assessments (n = 539) were imputed using the R package “mice” and replaced with the mode.
Participants were categorized as having normal spirometry if their FEV1, FVC, and FEV1/FVC were above the lower limit of normal (ie, Z score > −1.64) and were categorized as having abnormal spirometry if any one of those 3 measures was below or equal to the lower limit of normal.
Classification of Smoking Status and Respiratory Symptoms
Smoking history was categorized as never (not smoked 100 cigarettes in their lifetime), former (smoked at least 100 cigarettes but not in the past 30 d), and current (smoked at least 100 cigarettes and at least one cigarette in the past 30 d). Pack-year exposure was defined according to a previously described approach15 and categorized as < 10 or ≥ 10 pack-years. Using these 2 variables, smoking status was defined as never smoker, former smoker with < 10 or > 10 pack-years, and current smoker with < 10 or > 10 pack-years.
Current respiratory symptoms, including wheezing, shortness of breath (ie, dyspnea), and coughing, were obtained by self-reported questionnaire. Wheezing was represented by 3 questions: wheezing or whistling in your chest at any time within the last year, wheeze with mild-to-moderate exertion, and woken up with an attack of wheezing at any time within the last year. Shortness of breath was represented by 3 questions: become short of breath walking on flat surfaces, become short of breath climbing stairs or walking up a small hill, and had an attack of shortness of breath that came on during the day when you were at rest at any time within the last year. Coughing was represented by 2 questions: woken up with an attack of coughing at any time within the last year and usually coughed on most days within the last year. Answering yes to any one of these questions affirmed the presence of the respective symptom group, and if the question was refused or not answered, the respective symptom group was treated as missing data. Having any respiratory symptom was based on the presence of wheeze, dyspnea, or cough; for participants who did not report the presence of any symptoms, this dichotomous variable was assigned as missing if any of the individual symptom groups was also missing.
Frailty Index Calculation
Frailty was estimated using the frailty index approach16 based on 74 deficits related to chronic conditions, activities of daily living, depression, perceptions of health, satisfaction with life, body mass, and social participation as per previous work11,17 (Supplemental Table S1, see the supplementary materials at http://www.rcjournal.com). It is calculated as the proportion of deficits present relative to the total sum of deficits considered, ranging from 0–1 and is gamma distributed16,18; hence, increasing values represent worse health and greater risk of adverse outcomes. As an example, a person reporting 10 deficits would exhibit a frailty index of 0.135 (ie, 10 divided by 74). Frailty was defined as missing for any participant missing > 7 deficit variables (∼ 10%), which is similar or lower than other previously published studies,19-22 and participants with a frailty index of zero (n = 11) were assigned a value of 0.001 in order to accommodate gamma regression.
Covariates
The following factors were included as covariates in regression analyses given their known association with lung function, respiratory disease, and smoking status: age,23 sex,23 obesity,24 physical activity,25 diet,26 and income.23 Obesity was estimated by body mass index (BMI), obtained by measuring tape and stadiometer, and classified as underweight (< 18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (> 30 kg/m2); given that only a small number of participants was categorized as underweight (n = 109), they were combined with normal participants. Physical activity was operationalized using the Physical Activity Scale for the Elderly,27 a continuous measure in which a greater score indicates an overall greater amount of time spent per week performing activities such as walking, housework, and sports and recreational activities. Diet was evaluated based on participant fruit and vegetable consumption and defined as < 2 servings daily, 2–3 servings, 4–5 servings, or ≥ 6 servings; this information was captured within the SCREEN-II-AB assessment tool. Total household income was defined as annual earnings of < $50,000, $50,000–100,000, and > $100,000. Data for all factors were obtained by self-reported questionnaire; refusing or being unable to answer a given question was considered missing data.
Statistical Analysis
All continuous variables were summarized as the median and interquartile range and categorical variables as the count and frequency. Significance of variation across strata (ie, by smoking group or symptoms presence) was determined by ANOVA for continuous variables or chi-square test for categorial variables. To estimate the effect of smoking status and presence of any respiratory symptom on frailty, we used gamma regression (identity link), adjusting for age, sex, income, BMI, diet, and physical activity; observations with missing data in any of these variables were removed prior to analysis. The main effects of smoking and respiratory symptoms were determined using 3 models, 2 of which including smoking or respiratory symptoms and the third including both variables. We performed this analysis on the entire sample of participants as well as a subset of participants who did not report cardiopulmonary conditions: asthma, emphysema, chronic bronchitis, COPD, or chronic changes in lungs due to smoking, heart disease (including congestive heart failure), angina, myocardial infarction, an unstable heart condition in the past 3 months, or coronary artery bypass surgery (n = 14,176). Main effects for smoking were presented as the change in frailty (β coefficient and 95% CI) relative to never smokers and for respiratory symptoms relative to participants without any respiratory symptoms. We also tested whether there was a significant multiplicative interaction between smoking and respiratory symptoms in the aforementioned fully adjusted model.
To determine the proportion of the total effect of smoking status on frailty (ie, direct effect) that was mediated by respiratory symptoms (ie, indirect effect), we performed mediation analysis using the R package mediation.28 In this approach, adjusted gamma regression models were used to estimate both the direct and indirect effect of each smoking category on frailty, relative to never smokers, along with an adjusted logistic regression model to estimate the effect of smoking on the presence of respiratory symptoms. From these models, the proportion of effect mediated by respiratory symptoms (ie, ratio of the indirect to direct effect) can be determined, where a proportion of ≥ 1 (ie, 100%) represents complete mediation and < 1 represents partial mediation. This estimate is considered significant if the 95% CI does not cross 0. All analyses were performed in the R environment.
Results
Participant Demographics and Reported Respiratory Symptoms
Of the 21,293 participants included in our study, 18,478 (87%) had normal spirometry. Within this subset, 50% were never smokers, 43% were former smokers, and 7% were current smokers (Table 1). Current smokers, especially those with < 10 pack-years, tended to be younger than never and former smokers, and all smoking subgroups contained slightly more females except for former smokers with > 10 pack-years. Total household income tended to be lower in former and current smokers with > 10 pack-years, whereas diet (fruit and vegetable consumption) was markedly worse in current smokers with > 10 pack-years. Former > 10 pack-year smokers were more likely to be overweight or obese compared to the other categories and report less physical activity. As expected, FEV1 and FEV1/FVC were slightly lower in former and current smokers with > 10 pack-years, whereas the frailty index was higher. Unexpectedly, current smokers with < 10 pack-years exhibited the highest FEV1 and FVC, which is likely a product of their lower average age and higher physical activity. As a whole, these data indicate that there is significant heterogeneity among older adults in different smoking status categories.
Characteristics of Participants With Normal Spirometry
In our sample of participants with normal spirometry, 16%, 23%, and 26% reported wheeze, dyspnea, and cough, respectively, whereas 45% reported at least one symptom (Table 2). A similar proportion of never and former smokers with < 10 pack-years reported symptoms, and current smokers with < 10 pack-years were only slightly more likely to be symptomatic. As expected, former and current smokers with > 10 pack-years were much more likely to be symptomatic; in fact, 50% and 70%, respectively, reported at least one symptom. As shown in Table 3, when considered together, participants reporting at least one respiratory symptom tended to be older, female, and obese and had lower income, physical activity, FEV1, and FVC relative to those not reporting any symptoms. Further, symptomatic participants were more likely to be former or current smokers with > 10 pack-years and exhibited a median frailty index that was 0.04, or 44%, higher than those without symptoms. This difference exceeds the clinically meaningful difference of 0.03 that has been previously establish for the frailty index.29,30
Summary of Reported Respiratory Symptoms in Participants With Normal Spirometry
Characteristics of Participants With Normal Spirometry, Stratified by Respiratory Symptom Status
Frailty Differences According to Smoking Status and the Presence of Symptoms
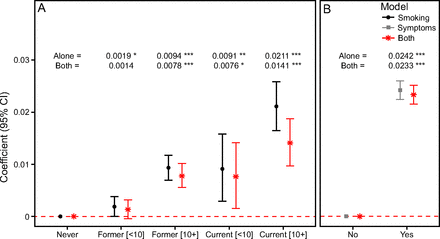
When modeled in the absence of respiratory symptoms, each smoking category was associated with significantly higher frailty relative to never smokers: former < 10 pack-years (adjusted coefficient 0.002, 95% CI 0.0001–0.0038), former > 10 pack-years (adjusted coefficient 0.009, 95% CI 0.007–0.012), current < 10 pack-years (adjusted coefficient 0.009, 95% CI 0.003–0.016), and current > 10 pack-years (adjusted coefficient 0.021, 95% CI 0.017–0.026) (Fig. 1; Supplemental Table S2, see the supplementary materials at http://www.rcjournal.com). In the absence of smoking, symptomatic participants also exhibited significantly higher frailty relative to those who were asymptomatic (adjusted coefficient 0.024, 95% CI 0.023–0.026]) (Fig. 1; Supplemental Table S2, see the supplementary materials at http://www.rcjournal.com), approaching the threshold of clinical meaningfulness (ie, 0.03). Interestingly, when both variables were modeled together, the effect of symptoms changed very little, whereas the effect of smoking on frailty decreased across all categories, the least of which for former smokers with > 10 pack-years (∼ 17% reduction) and the greatest for current smokers with > 10 pack-years (∼ 33%) (Fig. 1; Supplemental Table S2, see the supplementary materials at http://www.rcjournal.com).
Main effects of smoking status and respiratory symptoms on frailty in participants with normal spirometry. The results of 3 models are presented, all of which were adjusted for covariates: “smoking alone” and “symptoms alone” refer to models in which only smoking status and presence of symptoms were included, respectively, whereas “both” refers to a model including both variables. The regression coefficient and 95% CI, relative to never smokers or no respiratory symptoms, are presented as points and error bars; overlap with the red dashed line indicates no significant difference in frailty. Coefficient point estimates are also presented at the top of each plot, along with asterisks to denote significance; ***, P < .001; **, P < .01; *, P < .05.
Given that smoking can lead to the development of multiple cardiopulmonary disorders, which themselves can lead to respiratory symptoms and contribute to a higher frailty index, we performed a sensitivity analysis on participants that did not report any chronic respiratory or cardiovascular condition (n = 14,405 or 78% of the final sample). Although the magnitude of effects was lower, the trends observed within and between the smoking and respiratory symptoms variables changed very little (Supplemental Table S3, see the supplementary materials at http://www.rcjournal.com).
Respiratory Symptoms Partially Mediate the Effect of Smoking on Frailty
To further characterize the relationship between smoking and respiratory symptoms as correlates of frailty for participants with normal spirometry, we first tested for a possible interaction. However, no significant interaction between smoking and respiratory symptoms was observed, suggesting that the strength of association between respiratory symptoms and frailty does not change significantly according to smoking status and vice versa (Supplemental Table S4, see the supplementary materials at http://www.rcjournal.com).
Next, we investigated whether respiratory symptoms, which can be caused by smoking, mediate the association of smoking status with frailty (ie, smoking → symptoms → frailty). Our analysis indicated that symptoms partially mediated the association between frailty and smoking status, although the magnitude and significance depended on the smoking category in question. The effect of former and current smokers with < 10 pack-years relative to never smokers was mediated 21% (95% CI −53: 162%) and 14% (95% CI −2: 63%) by the presence of respiratory symptoms, respectively, but neither estimate was significant (Fig. 2). The effect of former and current smokers with > 10 pack-years, however, was significant, where 12% (95% CI 6: 19%) and 31% (95% CI 25: 40%) were found to be mediated by respiratory symptoms, respectively, (Fig. 2). This indicates that whereas there are significant independent effects of smoking status and respiratory symptoms on the frailty of older adults with normal spirometry, a significant proportion of the effect of smoking for those with > 10 pack-years is mediated through respiratory symptoms.
Analysis of the effect of smoking status on frailty, both direct and mediated by the presence of respiratory symptoms. (A) A directed acyclic graph illustrating the hypothesized relationship between smoking status, respiratory symptoms, and frailty. (B) The average causal mediation effect of respiratory symptoms (ACME; also known as the indirect effect), the average direct effect of smoking status (ADE), and the total effect of both smoking and symptoms on frailty for each smoking category relative to never smokers was estimated. The regression coefficient and 95% CI are presented. (C) For each contrast in B, the proportion of the total effect mediated by respiratory symptoms and 95% CI is presented. For both B and C, overlap with the red dashed line indicates that the respective estimate was not significant. ACME = indirect effect of smoking status. ADE = average direct effect of smoking status.
Discussion
Our data indicate that respiratory symptoms such as wheeze, cough, and dyspnea are very common in Canadian older adults with normal spirometry, regardless of smoking status. Approximately 40% of never smokers reported having at least one of these symptoms in the past year, whereas 50% and 70% of former and current smokers with > 10 pack-years reported these symptoms. We also found that those reporting respiratory symptoms were slightly older and more likely to be female, whereas former smokers were slightly older and those with > 10 pack-years more likely to be male, whereas current smokers tended to be much younger. Furthermore, those reporting respiratory symptoms were more likely to have lower income, fruit and vegetable consumption, and physical activity and be obese. Among smokers, these health risk factors tended to be more pronounced in those with > 10 pack-years. A recent study by Çolak and colleagues31 found similar trends: For adults with FEV1/FVC ≥ 0.70, the 37% that reported respiratory symptoms were slightly older and more likely to be female and exhibit health risk factors such as obesity, increased blood pressure, and chronic conditions. Im-portantly, symptomatic adults, especially former or current smokers, had a significantly higher odds of hospitalization and death, which would also suggest that levels of frailty were elevated in this subset. In the study by Woodruff and colleagues,3 symptomatic former or current smokers with preserved spirometry (FEV1/FVC ≥ 0.70 and FVC > lower limit of the normal range) were nearly as common as asymptomatic but exhibited significantly lower walking speed, an important component in the definition of frailty.32
The aforementioned work implies that even with normal spirometry older adults that exhibit respiratory symptoms, especially smokers, are more frail and at higher risk of adverse outcomes. Our results support this and suggest an interesting dependence between smoking status and respiratory symptoms. When regressed on smoking status or respiratory symptoms in separate models, multivariable analysis demonstrated that frailty increased with both smoking history and exposure, which has been previously shown.33,34 Similarly for respiratory symptoms, frailty was observed to be significantly higher in participants reporting any symptom over the past year, nearly to the same extent as current > 10 pack-years smokers relative to never smokers. However, when both smoking and respiratory symptoms were modeled together, the associations between smoking categories and frailty were reduced substantially, whereas the association with respiratory symptoms was nearly unchanged. Given that the associations between frailty and respiratory symptoms did not change appreciably among the smoking groups and were significant even if participants never smoked, we tested whether respiratory symptoms were a possible mediator of the association between smoking and frailty. Indeed, having at least one respiratory symptom partially mediated this relationship, although it was only significant for former and current > 10 pack-years smokers.
Whereas these findings indicate that respiratory symptoms are a very important correlate of frailty and an integral component of the harmful effects of smoking, the pathological mechanisms of their effects on frailty remain unclear; that being said, at least 2 explanations can be hypothesized. First, respiratory symptoms may be related to an underlying pulmonary condition that was not captured by spirometry or reported by the participant. This is supported by previous work showing that the incidence of hospitalization due to respiratory exacerbations (eg, asthma or COPD related) or mortality due to a respiratory condition is significantly greater for those exhibiting symptoms, even in never smokers with normal spirometry.31 Second, it is likely that respiratory symptoms have a measurable impact on health and well-being, independent of clinical or subclinical airway disease. This is supported by data from the European Community Respiratory Health Survey, which showed that respiratory symptoms are significantly associated with reduced health-related quality of life, even in the absence of asthma or COPD.35 The symptom-independent association of smoking with frailty in those with normal spirometry further supports the fact that there is no such thing as a healthy smoker.3,4
Our study featured a number of strengths and some limitations. First, our findings are based on data from a large, population-based study that allowed us to adjust for a broad array of important covariates in our modeling. Second, we were particularly conservative in our definition of both normal spirometry and smoking, the latter of which we categorized according to history and overall exposure. Lastly, instead of focusing on a single chronic condition or aspect of well-being as our outcome correlate, we employed a comprehensive frailty index, a sensitive measure of overall vulnerability to adverse outcomes in older adults. One of our major limitations was that we used a cross-sectional design, which did not allow us to infer causality in the associations we observed and relied on self-reported data, which are prone to response bias. Further, these associations, whereas close to what is considered a clinically meaningful difference, did not exceed them in adjusted analyses. Second, participants were not treated with bronchodilators prior to performing spirometry to classify them as normal or abnormal, which is not the typical approach in diagnosing obstructive or restrictive airway disease. Finally, although we were able to integrate both smoking history and exposure in our analyses, we were unable to incorporate the history of occupational exposures.
Conclusions
In summary, our results demonstrate that smoking history and exposure and respiratory symptoms are important correlates of frailty in older adults, even if they have normal spirometry or are free of self-reported cardiopulmonary disease. Respiratory symptoms appear to be especially important, exhibiting strong independent effects on frailty as well as partially mediating the association between smoking and frailty. Given that these associations are close to what are considered clinically meaningful differences in frailty, we believe that respiratory symptoms should be given particular attention when investigating possible airway disease in older adults and not be disregarded as a benign by product of aging.
Footnotes
- Correspondence: Chris P Verschoor MSc PhD, Health Sciences North Research Institute, 41 Ramsey Lake Road, Rm 32033, Sudbury, ON, Canada P3E 5J1. E-mail: cverschoor{at}hsnri.ca
The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://rc.rcjournal.com/.
This study was performed at Health Sciences North Research Institute, 41 Ramsey Lake Road, Sudbury, Ontario, Canada P3E 5J1.
This research was made possible by generous funding from the Ontario Thoracic Society and Ontario Lung Association and through data/biospecimens collected by the Canadian Longitudinal Study on Aging (CLSA). Funding for the CLSA is provided by the Government of Canada through the Canadian Institutes of Health Research under grant reference LSA 94473 and the Canada Foundation for Innovation.
This research has been conducted using the CLSA data set Baseline Comprehensive version 3.2, under application number 171012. The CLSA is led by Drs Raina, Wolfson, and Kirkland. The AB SCREEN-II-AB assessment tool is owned by Dr Heather Keller; use of the SCREEN-II-AB assessment tool was made under license from the University of Guelph.
- Copyright © 2021 by Daedalus Enterprises