Abstract
BACKGROUND: Methacholine bronchoprovocation or challenge testing (MCT) is commonly performed to assess airway hyper-responsiveness in the setting of suspected asthma. Nebulization is an aerosol-generating procedure, but little is known about the risks of MCT in the context of the ongoing coronavirus disease 2019 (COVID-19) pandemic. We aimed to quantify and characterize aerosol generation during MCT by using different delivery methods and to assess the impact of adding a viral filter.
METHODS: Seven healthy subjects performed simulated MCT in a near particle-free laboratory space with 4 different nebulizers and with a dosimeter. Two devices continuously sampled the ambient air during the procedure, which detected ultrafine particles, from 0.02–1 μm, and particles of sizes 0.3, 0.5, 1.0, 2.0, 5.0, and 10 µm, respectively. Particle generation was compared among all the devices, with and without viral filter placement.
RESULTS: Ultrafine-particle generation during simulated MCT was significant across all the devices. Ultrafine-particle (0.02–1 μm) concentrations decreased 77%–91% with the addition of a viral filter and varied significantly between unfiltered (P < .001) and filtered devices (P < .001). Ultrafine-particle generation was lowest when using the dosimeter with filtered Hudson nebulizer (1,258 ± 1,644 particle/mL). Ultrafine-particle concentrations with the filtered nebulizer devices using a compressor were higher than particle concentrations detected when using the dosimeter: Monaghan (3,472 ± 1,794 particles/mL), PARI (4,403 ± 2,948), Hudson (6,320 ± 1,787) and AirLife (9,523 ± 5,098).
CONCLUSIONS: The high particle concentrations generated during MCT pose significant infection control concerns during the COVID-19 pandemic. Particle generation during MCT was significantly reduced by using breath-actuated delivery and a viral filter, which offers an effective mitigation strategy.
Introduction
Methacholine bronchoprovocation or challenge testing (MCT) is a common procedure in the pulmonary function laboratory to assess airway hyper-responsiveness, usually in the context of suspected asthma. In 2017, the European Respiratory Society, with the endorsement of the American Thoracic Society, updated the recommended technical standards for MCT.1 These guidelines allowed for the use of dosimeters, breath-actuated nebulizers, or continuous nebulizers for MCT, with the requirement that manufacturers provide details of device output and particle size to allow for calculation of methacholine dose delivery. However, there is a paucity of data available with regard to the safety profile of these different dosing strategies, particularly in the context of the ongoing coronavirus disease 2019 (COVID-19) pandemic.
Nebulizer treatment is widely recognized to generate significant particle concentrations and has been classified by the World Health Organization as a possible aerosol-generating procedure.2 There is a growing body of evidence that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may have the potential to spread via airborne transmission.3-9 Many clinical studies demonstrated that SARS-CoV-2 RNA can be detected in air samples that surround individuals who are infected, but these studies did not document viable virus.10-13 However, Fears et al14 showed that aerosolized SARS-CoV-2 retained infectivity and virion integrity for up to 16 h. Therefore, MCT may pose significant infectious risk to health-care workers who administer this procedure and other individuals in close proximity. Our aim was to compare particle generation by using different methacholine delivery methods to inform our infection control and safety practices during the ongoing pandemic. We also examined the impact on particle generation of adding a viral filter to the nebulizer exhalation limb.
QUICK LOOK
Current Knowledge
There is growing evidence for the possibility of aerosol transmission of severe acute respiratory syndrome coronavirus 2. Methacholine challenge testing is an aerosol-generating procedure that may pose significant infectious risk during the coronavirus disease 2019 pandemic. There are no data available with regard to ambient particle generation during methacholine challenge testing with different delivery methods.
What This Paper Contributes to Our Knowledge
We compared particle generation by using various methacholine delivery methods and assessed the impact of adding a viral filter to the nebulizer exhalation limb. Particle generation was very high during methacholine challenge testing but can be minimized with breath-enhanced or breath-actuated delivery and the addition of a viral filter.
Methods
A prospective study was performed with 7 healthy volunteers. To accurately measure ultrafine-particle generation during MCT, testing was performed in a tightly sealed, nearly particle-free space designed to simulate a pulmonary function laboratory procedure room (188 × 229 × 305 cm [13,130 L]). The experimental space was connected in series to 2 portable 950 cfm fans with high-efficiency particulate air filter (HEPA) filtration model H1000V (Abatement Technologies, Suwanee, Georgia), which allowed particle concentrations within the experimental space to be reduced to < 1 ultrafine particle (0.02-1.0 µm) per mL before each testing series. Air flow was switched off during the tests.
Particle Counters
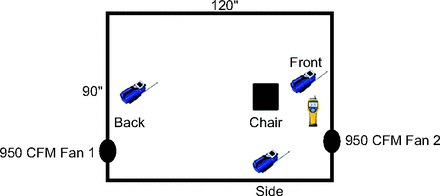
Continuous particle detection was performed at baseline and during simulated MCT by using 2 particle counters: an ultrafine-particle counter, P-Trak 8525 (TSI, Shoreview, Minnesota), for particles sizes between 0.02 and 1 µm; and Fluke 985 (Fluke, Everett, Washington), a 6-channel particle counter for particle sizes 0.3, 0.5, 1, 2, 5, and 10 µm. One P-Trak and one Fluke device were placed 12 inches in front of the seated subject, a second P-Trak device was placed to the side of the subject to quantify particle concentrations where a respiratory therapist would likely be standing during MCT, and a third P-Trak device was positioned in the back of the sealed space to quantify particle concentrations that have equilibrated throughout the room (Fig. 1).
Experimental design of particle-free laboratory space.
Methacholine Delivery Devices
Five MCT delivery methods were tested in this study. A KoKo dosimeter (KoKo, Longmont, Colorado) was used with the Hudson MicroMist nebulizer (Hudson RCI, Teleflex, Wayne, Pennsylvania). In addition, a simulated MCT was performed by using 4 nebulizers, including the Hudson MicroMist, Pari LC Plus (Pari Respiratory Equipment, Midlothian, Virginia), AeroEclipse II (Monaghan Medical, Plattsburgh, New York), and the AirLife MistyMax10 (Vyaire Medical, Mettawa, Illinois) (Table 1). All the nebulizers were used with the Ombra Compressor System (Trudell Medical, London, Ontario, Canada).
Nebulizer Device Characteristics and the Impact of Adding a Viral Filter on Ultrafine-Particle (0.02–1 µm) Concentrations
Study Protocol
The study was reviewed and approved by the Mayo Clinic Institutional Review Board (20 - 006779), and all the participants provided voluntary consent to participate. Each of 7 healthy subjects entered the sealed space and the ambient particles were cleared to < 1 ultrafine-particle/mL with the use of the HEPA fans, which were then turned off. The subjects then performed 1 min of unmasked tidal breathing, followed by simulated MCT informed by American Thoracic Society/European Respiratory Society guidelines.1 After each minute of tidal breathing, the subjects then performed 3 FVC maneuvers.
Typically, an FVC maneuver is performed at 30 s and 90 s after each level of the tidal breathing protocol, but 3 FVC maneuvers were chosen for the study protocol because individuals often exceeded 2 FVCs to achieve acceptable and repeatable results. Five rounds of 1 min of tidal breathing were performed to simulate the maximum, or worst case, particle concentrations during MCT. FVC maneuvers were performed through a MicroGard II PFT filter (Vyaire Medical), which is our standard practice during all pulmonary function laboratory testing. After completion of this testing protocol, the HEPA fans were turned on and ambient particle concentrations were reduced to restore the near particle-free environment.
Therefore, particle concentrations in the laboratory space were returned to < 1 ultrafine particle/mL between each device tested to ensure that the particles generated during testing for one device would not affect subsequent particle concentrations for the other devices tested. The testing protocol was then repeated with each device, with and without a viral filter (Hudson 1605 Main Flow Bacterial/Viral Filter, Hudson RCI) placed on the nebulizer exhalation limb. Strict randomization of testing order was not performed, but devices were not tested in a particular sequence. For safety purposes, saline solution was used rather than methacholine. The subjects were instructed to perform submaximal, normal tidal breaths, as is recommended in MCT to avoid the bronchoprotective effects of deep breathing.15-18 The AeroEclipse II was also tested by using the Monaghan bacterial/viral filter manufactured for use with this nebulizer. The AirLife MistyMax10 was not tested without a filter because it is manufactured with a viral filter in place.
Data Collection and Statistical Analysis
Particle concentration values were captured at 1-s intervals throughout each testing protocol. For graphic clarity, data were smoothed via 5–7 s averaging of the concentration gradient by using Prism version 8.2 (GraphPad Software, San Diego, California). Given that peak concentrations were highest near the end of simulated MCT, instantaneous particle concentrations were averaged over the final minute of testing to estimate maximum particle concentration. The reported ultrafine-particle concentrations were from the P-Trak device positioned in the “front” location (Fig. 1) because there were no significant differences in ultrafine-particle concentrations among the front, side, or back P-Trak devices. Particle concentration measurements when using the Fluke device was used to confirm these findings and to better characterize the range of particle size and concentration generated in the droplet cloud.
Particle concentrations were compared for each device with and without a viral filter by using paired sample t-tests with an alpha level of 0.05. The Friedman test was used to assess for any differences in particle concentrations during simulated MCT between the unfiltered devices and between the filtered devices. Post hoc analysis was performed by using the Conover test for pairwise comparisons of particle concentrations during each step. Alpha levels were set based on the Bonferroni correction for multiple pairwise comparisons. The alpha level was set at 0.008 for unfiltered device pairwise comparisons (6 comparisons) and 0.005 for filtered device pairwise comparisons (10 comparisons).
Results
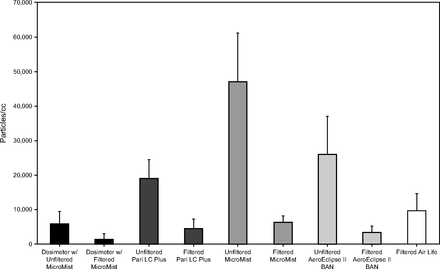
Mean cumulative particle counts measured by P-Trak (ultrafine-particle counter) during simulated MCT with each device are demonstrated in Figure 2. The mean concentration of ultrafine particles that ranged from 0.02 to 1 µm increased substantially during MCT testing with all the devices compared with unmasked tidal breathing (2.93 ± 1.62 particles/mL). Ultrafine-particle generation was lower with the KoKo dosimeter when using the unfiltered Hudson MicroMist (5,782 ± 3,738 particles/mL) and higher with unfiltered nebulizers when using the Ombra compressor: Pari LC Plus (19,059 ± 5,528 particles/mL), AeroEclipse II (25,722 ± 11,281 particles/mL), and Hudson MicroMist (47,078 ± 14,204 particles/mL). (Fig. 2)
Mean ultrafine-particle (0.02–1 µm) concentrations measured with the P-Trak particle counter during simulated methacholine bronchoprovocation or challenge testing (MCT) when using each device with and without a viral filter.
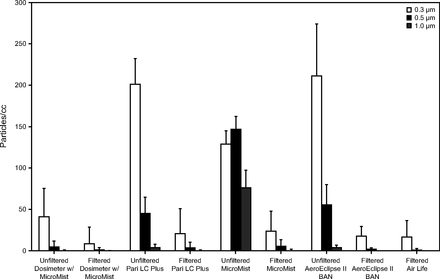
Mean particle concentrations measured when using the Fluke 985, which measures particles of size 0.3, 0.5, 1, 2, 5, and 10 µm. Particle concentrations > 1 µm were negligible (<1 particle/mL) and were excluded for graphic clarity.
The addition of a viral filter reduced ultrafine-particle generation significantly across all devices, ranging from 77% to 91% (Table 1). Overall, ultrafine-particle generation was lowest when using the KoKo dosimeter with filtered Hudson MicroMist (1,258 ± 1,644 particles/mL). Ultrafine-particle concentrations with the filtered nebulizer devices when using the Ombra compressor were higher than those seen with the dosimeter: AeroEclipse II (3,472 ± 1,794 particles/mL), Pari LC Plus (4,403 ± 2,948 particles/mL), Hudson MicroMist (6,320 ± 1,787 particles/mL), and AirLife MistyMax10 (9,523 ± 5,098 particles/mL). Ultrafine-particle generation was slightly higher with the Monaghan AeroEclipse II when using the Monaghan bacterial/viral filter (3,297 ± 1,365 particles/mL) than the Hudson 1605 filter (2,166 ± 1,199 particle/mL; P = .02).
There were significant differences in ultrafine-particle generation between the unfiltered devices (P < .001), which persisted when comparing only the unfiltered nebulizer devices powered by the Ombra compressor (P = .002). Similarly, there were also significant differences in ultrafine-particle generation between the devices with viral filters (P < .001), which persisted when comparing only the Ombra powered nebulizer devices (P = .007). The Conover test was used to perform pairwise comparisons between the filtered (Table 2) and unfiltered devices (Table 3). Small-particle concentrations predominated Fluke 985 measurements, with negligible measured concentrations of 2-, 5-, and 10-µm particles. Similar to ultrafine-particle concentrations (0.02–1 µm), the addition of a viral filter substantially reduced small particles (0.3, 0.5, 1.0 µm) measured by the Fluke device (Fig. 3).
Pairwise Comparisons of Ultrafine-Particle Generation Between Filtered Methacholine Delivery Devices by Using the Conover Test
Pairwise Comparisons of Ultrafine-Particle Generation Between Unfiltered Methacholine Delivery Devices by Using the Conover Test
Discussion
Current European Respiratory Society technical standards on bronchial challenge testing allow for the use of many different methacholine delivery methods, including the use of dosimeters and breath-actuated and continuous nebulizers.1 The ongoing COVID-19 pandemic has highlighted the significance of infection control and safety during pulmonary function laboratory testing, which underlines the importance of expanding on the considerations outlined by the 2017 guidelines. In response to design innovation and diversification, modern nebulizers are commonly defined in 3 categories.1 Constant-output nebulizers use a traditional T-piece and generate aerosol continuously throughout the breath cycle. Breath-enhanced nebulizers deliver more aerosol during inhalation by drawing ambient air through the nebulizer and release less air during exhalation by venting exhaled gas via an expiratory valve in the mouthpiece while the aerosol remains contained in the chamber. Dosimetric (or breath-actuated) nebulizers release aerosol only during inhalation.19
Constant output, or continuous, nebulizers have been criticized due to inefficient and unreliable drug delivery.20,21 Their continuous output results in the release of a significant proportion of nebulized medication from the T-piece during exhalation, with only a small percentage of medication delivered to the patient. The English-Wright nebulizer, previously the primary nebulizer recommended for use in the 1999 American Thoracic Society guidelines for bronchial challenge testing, has been associated with significant methacholine evaporative loss.22,23 A modern, high-efficiency nebulizer driven by a 50-psi gas source has been shown to deliver the same amount of drug in 12 s that the English-Wright nebulizer delivers in 2 min.24 Modern nebulizers, especially those with high-efficiency (breath-actuated or breath-enhanced), have been shown to increase the inhaled mass of medication and reduce ambient drug loss.21,25,26 These delivery methods may offer multiple safety advantages to pulmonary function laboratory personnel by reducing both exposure to nebulized methacholine and potentially infectious aerosolized particles.
We were able to quantify and characterize particle generation during MCT by using a variety of commercially available nebulizer devices, including a dosimeter (KoKo dosimeter) with the Hudson MicroMist, and the Ombra compressor powering a breath-actuated nebulizer (AeroEclipse II Breath-Actuated Nebulizer), breath-enhanced nebulizer (Pari LC Plus), and constant-output or continuous nebulizers (Hudson MicroMist and AirLife MistyMax10 with filter). These devices are commonly used within the Mayo Clinic Enterprise and were selected pragmatically to determine the device with the lowest particle generation and to inform our Enterprise methacholine protocol for use during and after the COVID-19 pandemic. In this study, particle generation was lowest with the use of a dosimeter, even when paired with a constant output nebulizer (unfiltered Hudson MicroMist), which was associated with the generation of the highest particle concentrations. Particle generation increased as expected based on nebulizer design and categorization, with progressively increasing particle concentrations seen with the breath-actuated nebulizer (AeroEclipse II Breath-Actuated Nebulizer), then the breath-enhanced nebulizer (Pari LC Plus), and highest with the continuous nebulizers (Hudson MicroMist and AirLife MistyMax10 with filter).19
Ultrafine-particle concentrations were 3 to 4 orders of magnitude higher than those previously measured by our group during peak flow testing (1–4 particles/mL).27 In addition, ultrafine-particle counts were ∼4 orders of magnitude higher than particle concentrations measured during unmasked tidal breathing, in both our previous work27 as well as the current study. This work demonstrated significant predominance of ultrafine particles that ranged from 0.2 to 1 µm, which are more likely to aerosolize and remain suspended in the air compared with larger particles (>5 µm), which have a higher tendency to settle on nearby surfaces. Therefore, the very high ultrafine-particle generation during MCT could pose a significant infectious risk and may warrant the use of enhanced protective strategies compared with other forms of pulmonary function testing.
The infection control strategies used at Mayo Clinic’s pulmonary function laboratory during the COVID-19 pandemic involve 4 important approaches. Although the community incidence of COVID-19 remains significant, we have used a targeted COVID-19 testing strategy to minimize the pre-test probability of patients who are asymptomatic or presymptomatic when presenting for pulmonary function laboratory testing. Our study focused on the second step in infection control mitigation: minimization of particle generation. Analysis of these results suggested that the use of breath-actuated or breath-enhanced methods of methacholine delivery produced significantly less aerosol, which reduces both occupational exposure and infectious risk. Several studies demonstrated that respiratory therapists have an increased risk of developing asthma compared with other medical professionals.28-30 Our study demonstrated that the addition of a viral filter could play an important role in further reducing aerosol production and improving MCT safety.
The third step in our infection control mitigation strategy involves enhancing containment and clearance of aerosols produced during MCT and other pulmonary function laboratory procedures. There are numerous factors that influence the particle cloud generated during MCT, including but not limited to the surrounding temperature and humidity, and the size, physical features, and air exchange characteristics of the testing room.31-33 An understanding of the ventilation in the clinical testing environment is also necessary because the number of air changes per hour would determine when the room could safely be used again. In our pulmonary function testing laboratory, the air changes per hour has been increased to 11–15 per hour to enhance clearance, and we have chosen to close our procedure room after each MCT procedure for sufficient time to allow 99.9% airborne contaminant removal.33 This is consistent with the 2017 European Respiratory Society recommendations for MCT, which recommend at least 2 air changes per hour and suggest other “optional methods to reduce methacholine exposure, including low resistance exhalation filters, a laboratory fume hood, supplemental local exhaust ventilation, and/or HEPA room air cleaners.”1 The final step in this approach involves increasing protective measures, for example, personal protective equipment. Given the very high number of ultrafine particles generated during MCT, we have chosen to use modified airborne and contact precautions during testing. Current protective measures include the use of a fit-tested N95 mask or powered air purifying respirator by health-care workers who administer MCT, in addition to protective eyewear, gown and gloves, and enhanced cleaning protocols.
Limitations and Future Directions
There were several limitations to this study. First, most commercially available particle counters have a small inherent measurement error for absolute particle concentration. However, given that multiple measurements were made with the subjects serving as their own internal control across each device, it was unlikely that a systematic measurement error impacted our results and conclusions. Second, we were unable to assess particle composition and other biologic properties that affected the risk of aerosolization. Third, the use of only healthy volunteers may limit the generalizability of the results. In addition, small-particle generation alone may not be able to serve as a reliable surrogate to determine infectious risk. Another important consideration is the potential impact of a viral filter on the delivery of inhaled microparticles during MCT or other procedures that involve nebulization. As the viral filter becomes saturated over the course of the nebulization, the impact on the delivered dose of methacholine is unclear. The impact of a viral filter on dose delivery of inhaled microparticles is not well described in the literature and warrants further investigation.
In this study, inspiratory flow was not measured with or without the viral filter in place. It is possible that variability in individual inspiratory flows could have affected particle generation. One study compared the effect of an unregulated high inspiratory flow (66–212 L/min) compared with a regulated low flow (20–35 L/min) and demonstrated that nebulizer output was not greater with the higher flow but did lead to increased variability.34 In addition, given that the American Thoracic Society/European Respiratory Society standards for MCT1 do not recommend regulation of inspiratory flows during MCT, the lack of strict control and measurement of inspiratory flows in this study may actually increase the generalizability of the results. Future studies should include a higher sample size of patients, including those with underlying pulmonary disease, to assess if there are significant differences in aerosol generation among individuals most likely to undergo MCT as well as to evaluate whether the addition of a viral filter could impact methacholine dose delivery.
Conclusions
Breath-enhanced or breath-actuated delivery of methacholine as associated with significantly lower particle generation during simulated MCT than during continuous nebulization. High levels of particle concentration during MCT may pose a significant infectious risk during the COVID-19 pandemic and were significantly reduced with the addition of a viral filter.
Footnotes
- Correspondence: Alexander S Niven MD, Pulmonary Function Laboratory, Division of Pulmonary, Critical Care, and Sleep Medicine, Mayo Clinic, 200 First Street, SW, Rochester, MN, 55905. E-mail: niven.alexander{at}mayo.edu
The authors have disclosed no conflicts of interest.
- Copyright © 2021 by Daedalus Enterprises