Abstract
BACKGROUND: Although systemic corticosteroids (SCS) have long been used to treat patients with COPD exacerbation, the recommended dose remains controversial. We aimed to perform a meta-analysis and an indirect treatment comparison to investigate the efficacy and safety of different doses of SCS in subjects with COPD exacerbation.
METHODS: Studies were identified by searching different databases for randomized controlled trials that investigated the efficacy and safety of SCS with placebo in subjects with exacerbation of COPD. The different doses of SCS were assigned to low-dose (ie, initial dose ≤ 40 mg prednisone equivalent/d [PE/d]), medium-dose (initial dose = 40–100 mg PE/d, and high-dose (initial dose > 100 mg PE/d) groups. The indirect treatment comparison was performed between low-, medium-, and high-dose SCS groups.
RESULTS: Twelve trials with 1,375 participates were included. Compared to placebo, the risk of treatment failure was lower in the low-dose SCS groups (risk ratio 0.61 [95% CI 0.43–0.88], P = .007) and high-dose SCS groups (risk ratio 0.64 [95% CI 0.48–0.85], P = .002); the FEV1 was significantly improved in low-dose (mean difference 0.09 [95% CI 0.06–0.12], P < .001), medium-dose (mean difference 0.23 [95% CI 0.02–0.44], P = .036), and high-dose SCS groups (mean difference 0.09, [95% CI 0.03–0.15], P < .001, respectively). Regarding safety, the incidence of hyperglycemia was higher in high-dose SCS groups versus placebo (risk ratio 2.52 [95% CI 1.13–5.62], P = .02). The indirect comparison between low-, medium-, and high-dose SCS found that the risk of treatment failure and changes in FEV1 were similar between these doses of SCS.
CONCLUSIONS: This meta-analysis indicates that low-dose SCS (initial dose ≤ 40 mg PE/d) was sufficient and safer for treating subjects with COPD exacerbation, and it was noninferior to higher doses of SCS (initial dose > 40 mg PE/d) in improving FEV1 and reducing the risk of treatment failure. However, our findings need to be verified in head-to-head randomized controlled trials.
Introduction
COPD is a common, preventable, controllable, and treatable disease. Patients with COPD are often exposed to large amounts of harmful particles or gases, resulting in abnormal airways and alveoli, persistent respiratory symptoms, and air flow limitations.1 The psychological, mental, and economic burden of COPD often exceeds that of other diseases; meanwhile, quality of life and work productivity of individuals with COPD is frequently reduced.2,3 To date, the morbidity of COPD has been estimated to be about 11.7% worldwide, with approximately 3 million people dying annually due to COPD. COPD has become one of the global leading causes of death.4
For individuals with COPD, symptoms develop over time and activities of daily living gradually decrease, with exacerbations of the disease occurring in many patients.3 COPD exacerbations accelerate respiratory failure and increase mortality and relapse rates.5,6 It is noteworthy that a remarkable number of COPD exacerbation patients who require hospitalization are readmitted within 6 months of the primary treatment.7,8
Several drugs are recommended for patients with COPD exacerbation, including bronchodilators (eg, β2-agonists, long-acting muscarinic antagonists, theophylline), antibiotics, and inhaled or systemic corticosteroids.9-13 Systemic corticosteroids (SCS) are commonly prescribed to treat patients with respiratory conditions such as asthma and to reduce the risk of flare-ups of inflammatory conditions, including rheumatologic and autoimmune diseases, allergic reactions, and inflammatory bowel disease. Although SCS have long been used to treat patients with COPD exacerbation, there have been no head-to-head randomized controlled trials (RCTs) to directly compare the effect of different dosage regimens of SCS. In addition, there is currently no consensus on the standard dose and duration of SCS in the treatment of COPD exacerbation. The Japanese Respiratory Society recommend oral prednisolone 30–40 mg/d for 7–10 d in patients with COPD exacerbation.14 However, the Thoracic Society of Australia and New Zealand recommend a 5-d course of 30–50 mg oral prednisolone, which is adequate for the treatment of exacerbations of COPD.15 Similarly, the steroid regimen recommended by the Global Obstructive Lung Disease (GOLD) guideline is 40 mg/d oral prednisolone for 5 d.1 One systematic review compared different durations of SCS therapy for subjects with COPD exacerbation,16 but the corresponding daily doses were not assessed. Another systematic review that concentrated on high-dose versus low-dose systemic steroids for treatment of exacerbation of COPD failed to identify any randomized trials comparing different doses of SCS.17 In addition, Cheng et al18 performed a meta-analysis to compare the efficacy and safety of different doses of SCS with placebo via subgroup analysis of high-dose and low-dose SCS, but this approach might not clearly explain which dose is better. Therefore, indirect treatment comparison could be used to obtain more rigorous results. We aimed to compare the efficacy and safety of different doses of SCS in subjects with COPD exacerbation using meta-analysis followed by an indirect treatment–comparison technique.
Methods
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement,19 and it was registered in the International Database of Prospectively Registered Systematic Reviews (PROSPERO; CRD42019122267). The selection process of the present meta-analysis is illustrated in Figure 1.
Flow chart. RCT = randomized controlled trial.
Two reviewers performed a comprehensive literature search for RCTs investigating the effects of different doses of SCS in subjects with COPD exacerbation. The relevant terms were searched in PubMed, Embase, Cochrane Library, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov to retrieve studies available up to December 4, 2019. The following key words were used in the literature search: corticosteroids (glucocorticoid, hydrocortisone, prednisone, prednisolone, methylprednisone, methylprednisolone, dexamethasone, triamcinolone, beclomethasone, betamethasone, fluticasone), and exacerbations of COPD (acute or chronic). Only articles published in English or Chinese were included. The detailed search strategy is shown in the supplementary materials (available at: http://www.rcjournal.com).
The criteria were selected via the framework of patient problem, intervention, comparison, outcomes, and study. Briefly, studies that met the following criteria were included in the analysis: the included patient problem was diagnosed as exacerbation of COPD according to the GOLD criteria1; intervention was SCS with usual treatment (eg, antibiotics and inhaled bronchodilators); comparison was usual treatment (eg, antibiotics and inhaled bronchodilators) versus placebo; outcomes were changes in FEV1, treatment failure (eg, death from any cause or the need for intubation and mechanical ventilation, readmission because of COPD, the need for additional treatment), relapse after treatment (eg, treatment for new exacerbation, readmission or hospitalization for COPD), hospital length of stay (LOS), mortality, and adverse effects. The study included RCTs comparing the efficacy and safety of SCS versus placebo in subjects with COPD exacerbation. We excluded studies using the following criteria: subjects with complications including allergic rhinitis, pulmonary infarction, pulmonary encephalopathy, asthma, atopy, pneumoconiosis, and active tuberculosis; studies of inhaled corticosteroids; non-RCTs; and review articles or other types of article.
We assessed the quality of each included study according to the Cochrane Handbook for Systematic Reviews of Interventions.20 We assessed risk of bias according to the following items: random-sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective outcome reporting. Two reviewers independently assessed the risk of bias and resolved disagreements by discussion.
The following study characteristics were extracted by 2 reviewers from included studies: publication details (eg, title, authors, and year of publication); subjects (eg, sample size, mean age, gender, smoking history, and inclusion criteria); interventions (eg, type of SCS, administration route, dose, and duration); outcomes (eg, changes in FEV1, treatment failure, relapse after treatment, hospital LOS, mortality, and adverse effects).
The primary outcomes of the current meta-analysis were treatment failure and relapse after treatment. The secondary outcomes were changes in FEV1, hospital LOS, mortality, and adverse effects. Dosing categories were assigned as follows: low-dose (initial dose ≤ 40 mg prednisone equivalent/d [PE/d]), medium-dose (initial dose = 40–100 mg PE/d), and high-dose (initial dose > 100 mg PE/d) groups recommended in established criteria of the literature and guideline.1,21 The SCS equivalent doses were used according to each drug’s relative anti-inflammatory potency.22
Data Analysis
Review Manager 5.3 software was used to perform statistical analysis. Statistical heterogeneity was assessed using the chi-square test and I2 statistical values. Random-effects models were used for significant heterogeneity, represented by I2 values > 50%; otherwise, a fixed-effects model was utilized. For continuous outcomes (ie, changes in FEV1 and hospital LOS), mean differences (MDs) with corresponding 95% CIs were used as effective measures. The risk ratio (RR) with corresponding 95% CIs was calculated as an effective measure for dichotomous outcomes (ie, treatment failure, relapse after treatment, mortality, and adverse effects). For outcome indicators with large heterogeneity, sensitivity analysis was used to find the source of heterogeneity. The subgroup analysis was performed if the duration of SCS was ≤ 5 d to verify the efficacy of the short-duration regimen in subjects with COPD exacerbation according to GOLD.1
Indirect treatment comparison was performed according to Bucher et al.23 If the pooled effect estimate for each group was statistically different compared with the placebo, the RR or MD for each indirect comparison was calculated using ITC (Indirect Treatment Comparison, Version 1.0, Ottawa: Canadian Agency for Drugs and Technologies in Health) software. This indirect comparison was made through a common comparator (placebo group). The efficacy or safety between 2 SCS doses was considered significantly different if the 95% CI did not contain a RR = 1 or MD = 0.
Results
Characteristics and Risk of Bias of Included Studies
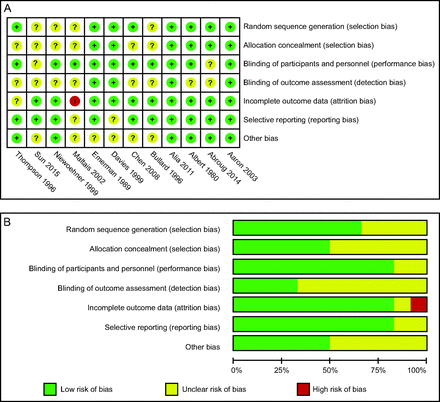
Of the 1,683 studies we retrieved from the aforementioned databases, we screened 12 RCTs24-35 with 1,375 subjects that met the inclusion criteria. Two studies24,35 included outpatients (assigned to low- and medium-dose SCS groups, respectively), and the other studies involved inpatients. Detailed features included in RCTs are presented in Table 1. In addition, risk of bias is shown in Figure 2. Eight studies described random-sequence generation, whereas in 4 studies the indicators were insufficient or unclear. Allocation concealment was adequate in 6 studies; however, the other 6 studies did not describe this aspect sufficiently or allocation concealment was not mentioned. Ten studies reported adequate blinding of subjects and investigators, whereas 2 studies did not state blinding of subjects and investigators. In addition, only 4 of the 12 included studies described blinding of outcome assessment. Ten studies were considered to have a low risk of attrition bias because no outcome data were missing or the data existed but did not have an impact on observed clinical effects; risk of bias was unclear for 1 study because it did not address the question of completeness, and 1 study was considered to have a high risk of bias due to incomplete outcome data, which had an impact on observed clinical effects. Selective outcome reporting did not appear in 10 studies, whereas it remained elusive in the other 2 studies. Finally, we did not identify other sources of bias in 6 studies, and they were unclear in the other 6 studies.
Characteristics of Enrolled Studies
A: Risk of bias summary for included studies, showing each risk of bias item for every included study. B: Risk of bias graph presenting each risk of bias item as percentages across all included studies.
Meta-Analysis Outcomes
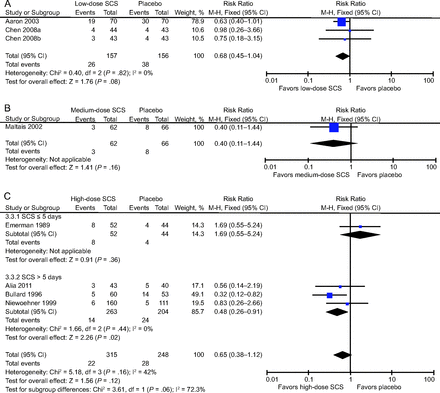
Table 2 shows details of the meta-analysis results of all outcomes. Overall, 12 studies mentioned the outcome of treatment failure. The risk of treatment failure was significantly lower in low-dose and high-dose SCS groups compared to placebo (RR 0.61 [95% CI 0.43–0.88], P = .007; RR 0.64 [95% CI 0.48–0.85], P = .002) (Fig. 3). However, the medium-dose SCS group and the high-dose SCS group with duration ≤ 5 d did not show significant difference of treatment failure versus placebo (RR 0.56 [95% CI 0.18–1.71], P = .31; RR 1.47 [95% CI 0.56–3.85], P = .43) (Fig. 3). When the outpatient studies were removed, the pooled effect estimates decreased slightly in the low-dose SCS groups, and the risk of treatment failure was then not significantly lower than placebo (RR 0.59 [95% CI 0.34–1.02] P = .057; see the supplementary materials at http://www.rcjournal.com). The pooled effect estimates slightly increased in the medium-dose SCS group but was still not significantly different than placebo (RR 0.76 [95% CI 0.29–1.98], P = .58; see the supplementary materials at http://www.rcjournal.com).
Pooled Effect Estimates of Studied Outcomes
Forest plots for the effect of different doses of SCS on treatment failure. A: Low-dose SCS versus placebo; (B) medium-dose SCS versus placebo; (C) high-dose SCS versus placebo. SCS = systemic corticosteroids.
There were 7 studies that reported the outcome of relapse after treatment, and the forest plot indicates that low-, medium-, high-dose SCS groups were all not significantly different versus placebo (RR 0.68 [95% CI 0.45–1.04], P = .08; RR 0.4 [95% CI 0.11–1.44], P = .36; and RR 0.65 [95% CI 0.38–1.12], P = .12, respectively) (Fig. 4). Nevertheless, subgroup analysis indicated that the incidence of relapse after treatment was lower in high-dose SCS group for > 5 d compared to placebo (RR 0.48 [95% CI 0.26–0.91], P = .02) (Fig. 4). When the outpatient study was removed, the direction of the pooled effect estimates did not change in the low-dose SCS group versus placebo (RR 0.86 [95% CI 0.33–2.28], P = .77; see the supplementary materials at http://www.rcjournal.com).
Forest plots for the effect of different doses of SCS on relapse after treatment. A: Low-dose SCS versus placebo; (B) medium-dose SCS versus placebo; (C) high-dose SCS versus placebo. SCS = systemic corticosteroids.
The effects of low-, medium-, high-dose SCS groups versus placebo on the changes in FEV1 are shown in Figure 5 and Table 2. The pooled effect estimates showed that the changes in FEV1 were not noted between high-dose SCS for ≤ 5 d and placebo (MD 0.1 [95% CI –0.02 to 0.21], P = .09); but there was significant difference between high-dose SCS for > 5 d (MD 0.09 [95% CI 0.03–0.15], P = .004), medium-dose SCS (MD 0.23 [95% CI 0.02–0.44, P = .036), and low-dose SCS (MD 0.09 [95% CI 0.06–0.12, P < .001) and placebo (Fig. 5). The pooled MD of low-dose SCS group remained unchanged when the outpatient study was removed (MD 0.09 [95% CI 0.06–0.12], P < .001), and only 1 study was left in the medium-dose SCS group (MD 0.14 [95% CI 0.13–0.16], P < .001; see the supplementary materials at http://www.rcjournal.com).
Forest plots for the effect of different doses of SCS on changes in FEV1. A: Low-dose SCS versus placebo; (B) medium-dose SCS versus placebo; (C) high-dose SCS versus placebo. SCS = systemic corticosteroids.
The low-dose SCS group exhibited decreased hospital LOS versus placebo (MD –1.21 [95% CI –2.24 to –0.19], P = .02) using a fixed effects model (I2 = 47%). For the medium- and high-dose SCS groups, there was only 1 study in each group, and the SCS did not show significant differences compared to placebo (Table 2). Meanwhile, SCS groups did not show significant difference in mortality compared to placebo (Table 2; see the supplementary materials at http://www.rcjournal.com).
The incidence of hyperglycemia in low-dose and medium-dose SCS groups did not show significant difference versus placebo (Table 2). However, the high-dose SCS group had a significantly increased incidence of hyperglycemia versus placebo (RR 2.52 [95% CI 1.13–5.62], P = .02) using a fixed-effects model (I2 = 47%). For increasing the risk of gastrointestinal bleeding, each SCS group was not statistically different compared to placebo (Table 2; see the supplementary materials at http://www.rcjournal.com).
Table 3 shows details of the results of indirect treatment comparison. For each outcome, the indirect treatment comparison was performed when there were at least 2 SCS groups or subgroups in which the pooled effect estimates were statistically different compared to the placebo. Thus we indirectly compared treatment failure and changes in FEV1 between SCS groups (or subgroups) using placebo as the common comparator. For treatment failure, there was no significant difference between the low-dose group and the high-dose group (RR 0.95 [95% CI 0.62–1.47], P = .92), nor was there a significant difference between the low-dose group and the high-dose for > 5 d subgroup (RR 1.07 [95% CI 0.67–1.72], P = .89). For changes in FEV1, the low-, medium-, high-dose groups and high-dose for > 5 d subgroup were indirectly compared, and there was no significant difference in any comparison with the P values ranging from .47 to .99 (Table 3).
Indirect Comparisons Between Low, Medium, and High Doses of SCS in COPD
Discussion
Our meta-analysis investigated the safety and efficacy of different doses of SCS in subjects with COPD exacerbation. The results of this meta-analysis indicate that low-dose SCS (ie, initial dose ≤ 40 mg PE/d) was a sufficient and safer treatment for subjects with COPD exacerbation, and it was noninferior to higher-dose SCS (ie, initial dose > 40 mg PE/d) for improving FEV1, reducing the risk of treatment failure, and shortening hospital LOS.
Over the past several decades, SCS drugs have been approved for the clinical treatment of COPD exacerbation. However, the most appropriate dose for patients with exacerbation of COPD remains elusive. Because there are no direct RCT comparisons between various doses of SCS, we used indirect comparison techniques to explore the efficacy and safety of different doses of SCS to treat subjects with a COPD exacerbation. In this meta-analysis, we investigated the safety and efficacy of different doses of SCS in subjects with exacerbation of COPD. The enrolled studies were assigned to low-dose, medium-dose, and high-dose SCS groups. The grouping of SCS dose regimens that we used were different from those used in the study by Cheng et al.18 They defined low-dose SCS as an initial dose < 80 mg PE/d, but this may not be precise due to the wide range of dosages. For example, combining outcomes from the study performed by Maltais et al,32 in which 60 mg prednisolone/d was used, with outcomes from other RCTs in which a dose regimen < 40 mg prednisolone/d was used might impact the pooled effect estimates. Therefore, we assigned the studies in which doses were < 40 mg prednisolone/d to the low-dose SCS group according to GOLD guidelines, which recommend 40 mg prednisolone/d for COPD exacerbation.1 We defined the high-dose SCS as an initial dose > 100 mg PE/d based on a previous review.21 Compared to placebo, high-dose SCS with a duration > 5 d was associated with lower risk of treatment failure or relapse and improved FEV1; however, this treatment significantly increased the incidence of hyperglycemia, which was demonstrated in a previous cohort study.36 Medium-dose SCS improved FEV1 versus placebo, but it did not show superiority in other outcomes. Interestingly, only low-dose SCS significantly shortened the hospital LOS versus placebo, which was consistent with previous observational studies.37,38 Generally, COPD severity in outpatients differed from that of inpatients. When outpatient studies were removed, the pooled effect estimates of changes in FEV1 and relapse remained largely unchanged; the risk of treatment failure of the low-dose SCS group was no longer significantly different from placebo, but showed a trend (P = .057) that should be interpreted with caution.
The results of our indirect comparisons indicate that there was no significant difference between various SCS doses and treatment failure or changes in FEV1. Meanwhile, the SCS subgroup with SCS > 5 d did not show superiority compared to other SCS groups in reducing the risk of treatment failure and improving FEV1. Leuppi et al39 performed an RCT and reported that, in the long-term SCS group, mean cumulative prednisone dose was statistically higher, but no significant difference was noted in time to death or in the combined end point of exacerbation and recovery of lung function compared to short-term SCS. The results of this RCT are consistent with ours. The current meta-analysis results were also accordant with previous observational studies.36-38 High-dose and long-term SCS should be used with caution due to the risk of hyperglycemia, and these regimens did not show superiority in improving lung function or prognosis compared with lower dose SCS.
A previous Cochrane review16 reported that shorter courses of SCS (ie, ∼ 5 d) did not lead to worse outcomes compared with longer courses (ie, 10–14 d). The RECUT trial, which is currently recruiting subjects, is designed to explore whether a 3-d treatment with orally administered SCS is noninferior to 5-d treatment in subjects with COPD exacerbation, and whether total corticosteroid exposure can be reduced by shorter therapy duration.40 In the current investigation, there is a trend to reduce the duration of SCS courses in subjects with COPD exacerbation. In the meantime, to reduce exposure of patients with COPD exacerbation to SCS, the daily dose should also be considered. A current RCT (NCT01742338) has been designed to perform a direct, head-to-head comparison of different doses of corticosteroids to determine whether a high-dose corticosteroid regimen in subjects with COPD exacerbations is associated with better clinical outcomes and at acceptable risk of adverse effects compared to a low-dose corticosteroid regimen, but that study is still recruiting. In this meta-analysis, we noted that an initial SCS dose ≤ 40 mg PE/d was noninferior for reducing the risk of treatment failure and for improving FEV1 compared to a higher dose of SCS, and it appears sufficient to treat patients with COPD exacerbation. However, an indirect treatment comparison is not as precise as direct research. Therefore, RCTs evaluating various SCS doses in COPD exacerbation are needed to define the most appropriate dose for patients with COPD exacerbation and to promote the rational clinical use of SCS.
This meta-analysis has several limitations. First, the common comparator (in this case, placebo) should be similar across the included RCTs; however, the characteristics (eg, the follow-up protocols) of the included studies may not be totally consistent. Second, only outcomes (ie, changes in FEV1 and treatment failure) were indirectly compared. Due to the lack of pooled statistically significant data in each group, we could not detect differences in other outcomes (eg, relapse after treatment, hospital LOS, mortality, and adverse effects) between various doses of SCS to treat COPD exacerbation. Third, there was significant heterogeneity in some outcomes, while the effect estimates remained stable after removing 1 study at a time. Fourth, some studies in this review had relatively small sample sizes, although no significant risk of bias was noted. The study with small sample size likely lacks sufficient statistical power to detect true positive associations; however, pooling these high-quality studies could achieve reliable results.
Conclusions
This meta-analysis indicates that low-dose SCS (ie, initial dose ≤ 40 mg PE/d) is sufficient and safer for treatment of subjects with COPD exacerbation, and that it was noninferior to higher-dose SCS (ie, initial dose > 40 mg PE/d) in improving FEV1, reducing the risk of treatment failure, and shortening hospital LOS. However, further RCTs that directly compare the efficacy and safety of different SCS doses in COPD exacerbation are required.
Footnotes
- Correspondence: Guojun Wang MSc, Department of Pharmacy, The Affiliated Hospital of Southwest Medical University, 25 Taiping St, Luzhou, Sichuan 646000, China. E-mail: renren333{at}126.com
Mr Pu and Mr Liu are co-first authors.
The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2021 by Daedalus Enterprises