Abstract
BACKGROUND: It is unknown whether lung mechanics differ between patients with pediatric ARDS and at risk for ARDS. We aimed to examine the hypothesis that, compared to ARDS, subjects at risk of ARDS are characterized by higher end-expiratory lung volume (EELV) or respiratory system compliance (CRS) and lower distending pressure (stress) applied on the lung or parenchymal deformation (strain) during mechanical ventilation.
METHODS: Consecutively admitted subjects fulfilling the PALICC ARDS criteria were considered eligible for inclusion in this study. A ventilator with an integrated gas exchange module was used to calculate EELV, CRS, strain, and stress after a steady state had been achieved based on nitrogen washout/washin technique. All subjects were subjected to incremental PEEP trials at 0, 6, 12, 24, 48, and 72 h.
RESULTS: A total of 896 measurements were longitudinally calculated in 32 mechanically ventilated subjects (n = 15 subjects with ARDS; n = 17 subjects at risk for ARDS). EELV correlated positively with strain or stress in the ARDS group (r = 0.30, P < .001) and the at risk group (r = 0.60, P < .001). CRS correlated with strain (r = 0.40, P < .001) only in subjects at risk for ARDS. EELV increased over time as PEEP rose from 4 to 10 cm H2O in subjects with ARDS (P = .001). In the at risk group, EELV only increased at 48 h (P = .001). Longitudinally, CRS (P = .001) and EELV (P = .002) were lower and strain and stress were higher in subjects with ARDS compared to those at risk for ARDS (P = .002), remaining within safe limits. Strain and stress increased by 24 h but declined by 72 h in subjects with ARDS at a PEEP of 4 cm H2O (P = .02). In the at risk group, strain and stress declined from 6 h to 72 h at a PEEP of 10 cm H2O (P = .001).
CONCLUSIONS: Longitudinally, CRS and EELV were lower and strain and stress were higher in subjects with ARDS compared to subjects at risk for ARDS. These parameters behaved differently over time at PEEP values of 4 or 10 cm H2O. At these PEEP levels, strain and stress remained within safe limits in both groups.
- compliance
- end-expiratory lung volume
- stress
- strain
- acute respiratory distress syndrome
- at risk
- pediatric
- washin/washout technique
Introduction
Pediatric ARDS causes significant morbidity and mortality in infants and children.1 ARDS is characterized physiologically by hypoxemia and reduced lung volumes and respiratory system compliance (CRS), although the primary metric to define disease severity in pediatric ARDS is the oxygenation index (OI). The new definitions of pediatric ARDS and pediatric patients at risk for ARDS, recently proposed by Pediatric Acute Lung Injury Consensus Conference (PALICC), have mainly focused on the true epidemiology and risk stratification of the disease.2,3 Although the 2 ARDS groups only differ by the OI limits (borders of 4), they have never been studied with regard to basic lung mechanics differences.
End-expiratory lung volume (EELV) is the functional residual capacity (FRC) plus lung volume increased by the applied PEEP. Increased PEEP levels lead to either increased EELV due to alveolar recruitment or distention of already ventilated alveoli, which results in lung injury in patients with low lung recruitability. The ratio of inflated tidal volume (VT) to FRC, defined as volumetric strain, causes physical lung deformation. The corresponding changes in transpulmonary pressure at end inspiration, defined as stress, are directly applied to the alveolus.4 Strain and stress should be readily estimated and ventilator settings appropriately individualized to properly set the mechanical breath and further eliminate the possibility of ventilator-induced lung injury.5
There is little evidence indicating optimal ventilation practices for pediatric ARDS, and ventilation practices are mainly based on institutional preferences, personal beliefs, and clinical data extrapolated from adult patients with ARDS. Low VT has been shown to minimize tidal over-distention (volutrauma), and higher PEEP levels are used to reduce cyclic closing and reopening of alveoli (atelectrauma).6 Evidence derived from adult cohorts, however, indicates that low VT ventilation may not limit injury from repetitive alveolar opening and closing, and lung recruitment with high PEEP may increase mortality compared to low PEEP.7 Furthermore, age-related and disease-dependent factors may influence lung mechanics unpredictably in patients with ARDS or those at high risk of progression to ARDS. Accordingly, a fundamental point for setting the ventilator would be an approximate estimation of the age-related and disease-dependent characteristics or outcomes of mechanical ventilation, both beneficial and harmful, that vary over time.8,9
User-friendly methods of bedside incremental PEEP titration, balancing alveolar recruitment against overdistention, might personalize PEEP and help optimize lung recruitment and homogeneity of ventilation in pediatric patients.10,11 Measuring EELV when PEEP is applied might be a better indicator than CRS to assess which patients may benefit from recruitment strategies, given that ARDS is characterized by a major loss of lung volume. This problem has recently signified the need for clinical and experimental studies to better understand the use and effects of mechanical ventilation in pediatric patients with or without lung injury.12
Using a nitrogen washout/washin technique, previously shown to correlate well with computed tomography scanning,13 we recently reported that daily trends of lung mechanics might be monitored noninvasively based on real-time different responses to PEEP changes of subjects without lung injury or with ARDS.14 Based on these preliminary results, we hypothesized that subjects at risk for ARDS would be longitudinally characterized by higher CRS or EELV but lower distending pressure (stress) or parenchymal deformation (strain) compared to subjects with ARDS. Also, we hypothesized that lung mechanics responses to time effects or PEEP changes might differ unpredictably between subjects with ARDS and subjects at risk for ARDS. Accordingly, using the nitrogen technique, we aimed to compare longitudinal 72-h trends and changes of lung mechanics in the 2 pediatric ARDS groups. The main objective was to assess similarities and differences of EELV, CRS, stress, and strain between subjects with ARDS and those at risk for ARDS when applying incremental PEEP on the first and second ARDS day. Secondary objectives were to compare the relationships between EELV, CRS, stress, and strain, as well as the effects of time on EELV, CRS, stress, strain, and safety levels at either low (4 cm H2O) or high (10 cm H2O) PEEP levels between the pediatric subjects with ARDS and pediatric subjects at risk for ARDS.
QUICK LOOK
Current Knowledge
Little evidence supports optimal ventilation practices for pediatric patients with ARDS, which are mainly based on institutional preferences, personal beliefs, and clinical data extrapolated from adults. Pediatric patients with ARDS and those at risk for ARDS present similarly with acute-onset pulmonary parenchymal disease within 7 d of a presumed clinical insult and only differ in terms of oxygenation index limits.
What This Paper Contributes to Our Knowledge
Using a noninvasive nitrogen washout/washin technique, based on real-time responses to PEEP changes, our prospective study indicated that, during a 72-h period, respiratory system compliance and end-expiratory lung volume were lower, strain and stress were higher, and their interrelations were weaker in subjects with ARDS compared to subjects at risk for ARDS. These parameters behaved differently over time at PEEP values of 4 or 10 cm H2O. At all time points, strain and stress remained within safe limits in both groups.
Methods
Study Design
This was a prospective longitudinal study of mechanically ventilated children age < 18 y with lung injury admitted to a multidisciplinary 7-bed pediatric ICU at the University Hospital, University of Crete, Heraklion, Greece, between June 2018 and December 2019. This study was conducted in accordance with the amended Declaration of Helsinki. The local institutional review board approved the protocol (1152/30/01–11-2017), and written informed consent was obtained from subjects’ guardians before inclusion in this study. All data were de-identified.
Subjects
Consecutively admitted patients with lung injury were considered eligible for inclusion in this study if they were anticipated to be mechanically ventilated for > 48 h. The recruited subjects were stratified into the ARDS group or the group at risk for ARDS at a matched baseline PEEP of 5 cm H2O, which is the lowest level used to define ARDS according to both the Berlin15 and the PALICC pediatric ARDS criteria.1 Patients were recruited if they met the following PALICC diagnostic criteria for the first time within the previous 24 h: new infiltrate(s) consistent with pulmonary parenchymal disease; timing within 7 d of a clinical insult; ARDS was defined as a minimum level of hypoxemia based on mechanical ventilator support (OI ≥ 4; mild ARDS is defined as an OI of 4–8, moderate ARDS as an OI of 8–16, and severe ARDS as an OI > 16), whereas the at risk group was defined by OI < 4.1 The exclusion criteria were perinatal related lung disease, severe hemodynamic instability (defined as an increase in vasoactive drugs dosages in the last 6 h to increase mean arterial pressure [MAP] or cardiac index measured with pulse contour cardiac output), thoracic deformations, chronic respiratory disease, and technique accuracy issues ( > 0.60 or air leaks > 10%). Subjects in the supine position were mechanically ventilated in the volume control mode (Carescape R860, GE Healthcare, Madison, Wisconsin). When available, and since lung recruitment is known to result in hemodynamic compromise,16 simultaneous invasive MAP and cardiac index measurements were included in the analyses because hemodynamics should be closely monitored as PEEP is increased. Pediatric Logistic Organ Dysfunction (PeLOD) and Pediatric Risk of Mortality (PRISM III) scores were calculated for each subject.
> 0.60 or air leaks > 10%). Subjects in the supine position were mechanically ventilated in the volume control mode (Carescape R860, GE Healthcare, Madison, Wisconsin). When available, and since lung recruitment is known to result in hemodynamic compromise,16 simultaneous invasive MAP and cardiac index measurements were included in the analyses because hemodynamics should be closely monitored as PEEP is increased. Pediatric Logistic Organ Dysfunction (PeLOD) and Pediatric Risk of Mortality (PRISM III) scores were calculated for each subject.
Study Protocol
All subjects were sedated with midazolam (0.1 mg/kg/h) and fentanyl (1 μg/kg/h) or remifentanil (0.025 μg/kg/min) intravenously, titrated as indicated, with no spontaneous breathing efforts. Ventilator settings before and after completing the test were at the discretion of the critical care team, which drew blood gases as clinically indicated. Any clinical intervention of the research team with the child’s existing ventilatory strategy including PEEP before and after the study’s duration was out of scope for this research. Ventilation was set in volume control mode, and VT was set at 6 mL/kg predicted body weight and remained unchanged during the entire PEEP trial.
The PEEP INview software of the ventilator, a noninvasive in-built application for the indirect measurement of EELV at the bedside using the modified nitrogen washout/washin technique with an integrated gas exchange module (E-COVX-00, Carescape R860, GE Healthcare), was used to calculate EELV. The initially calculated EELV at 0 PEEP was considered to be the measurement of FRC. Four different PEEP levels were selected according to an integrated ascending PEEP trial (ie, 4, 6, 8, and 10 cm H2O). At each PEEP level, the EELV measurements were automatically repeated (washout and washin) within a single procedure, using a 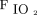 step change of 0.2. EELV was measured at each PEEP step only after a steady state lasting at least 10 min had been achieved automatically to ensure nitrogen washout/washin. At the end of the procedure, numeric values of the different EELVs and CRS were displayed on the screen. Because lung volumes are affected by height (ie, lung volume is proportional to body size), EELV and CRS were normalized to body mass index for comparison.
step change of 0.2. EELV was measured at each PEEP step only after a steady state lasting at least 10 min had been achieved automatically to ensure nitrogen washout/washin. At the end of the procedure, numeric values of the different EELVs and CRS were displayed on the screen. Because lung volumes are affected by height (ie, lung volume is proportional to body size), EELV and CRS were normalized to body mass index for comparison.
Tests were performed upon ARDS diagnosis (baseline, or time point 0) and at predetermined time points at 0, 6, 12, 24, 48, and 72 h. For comparison, measurements at baseline and at 48 h were defined as day 1 and day 2 measurements, respectively. Hemodynamic parameters, namely heart rate, MAP, and cardiac index were simultaneously and continuously recorded. Blood gases were drawn before and after each time point per study protocol.
Calculations
Calculations used were adapted from the PEEP-step method validated during general anesthesia.17 We calculated static strain and dynamic strain, terms introduced by Protti et al18 to describe the energy applied to the lungs. Static strain, which describes the lung tissue deformation due the applied PEEP energy at each setting, was determined as the ratio of PEEP volume to FRC, where PEEP volume is the difference between EELV and FRC at a given PEEP level. Strain dynamic, which describes the dynamic process of the lung deformation due to VT energy that is cyclically applied to the lungs, is the ratio of VT to FRC. Lung stress, which describes the distribution of forces due to PEEP and VT resulting in strain, was calculated as strain × k; in humans, specific lung elastance (k) has been calculated in clinical studies to be nearly 13.5 cm H2O.19,20 Reported safety levels for static strain (< 1.5) and static stress (< 20 cm H2O) were used to examine the safety of the 95% CI of recorded values at all PEEP levels.19 Routinely used calculations included driving pressure, calculated as end-inspiratory plateau pressure minus total PEEP, and OI, calculated as (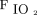 × P̅aw × 100)/
× P̅aw × 100)/ .
.
Statistical Analysis
GPower 3.1,21 which was used to calculate the study sample size, determined that for repeated measures between groups for 4 PEEP levels, there is an 82% chance of correctly rejecting the null hypothesis of no difference between the ARDS group and at risk for ARDS group with a total of 25 subjects. Looking for a cross-sectional predicted difference in means between the 2 groups of 5 for lung stress on day 2 (assuming a pooled standard deviation of 7 units, 95% CI, power 80%, and a 2-sided 5% level of significance), a total sample size of 32 subjects (16 for each group) was calculated. The > 800 measurements in this study cover the estimated total degrees of freedom of 243 measurements calculated by the statistical software (effect size = 0.25, α = 0.05, power [ie, 1 – β error probability] = 0.80). This effect size is reliable (ie, large) in predicting real differences between the 2 group means divided by the pooled standard deviation. The Levene test for the homogeneity of group variances was used to determine the data distribution from measured variables. All continuous parameters are presented as median (interquartile range). Categorical variables were expressed as frequencies. The Mann-Whitney U test or the Kruskal-Wallis and Dunn-Bonferroni pairwise comparisons were used to perform comparisons between groups, as appropriate. The generalized estimating equations procedure was used to extend the generalized linear model (GLM/GEE) to allow for comparison of repeated measurements between the 2 groups. A mixed-model, repeated-measures, analysis of variance followed by post hoc tests was also used to test for group versus time interaction. The Mauchly sphericity test, along with the Greenhouse-Geisser correction, was used to validate the results of mixed repeated measures. Pairwise comparison was done with Bonferroni post hoc tests (intragroup and intergroup) to identify the differences in EELV, CRS, strain, and stress levels between the study groups (between-subjects) within time intervals and repeated PEEP levels (within-subjects). Categorical variables were compared with Pearson chi-square or Fisher exact tests as appropriate. Correlations between 2 continuous variables were determined using bivariate correlations (Pearson or Spearman coefficients). Using the Fisher r-to-z transformation, a value of z was calculated to assess the significance of the difference between the correlation coefficients of the two pediatric ARDS groups. A 2-sided significance level of .05 was used for statistical inference. All statistical analyses were performed using the SPSS 25.0 (SPSS, Chicago, Illinois).
Results
Subjects
After having screened 172 consecutively admitted patients anticipated to be mechanically ventilated for > 48 h, 140 were excluded because they did not meet inclusion criteria (noninvasive ventilation, n = 66; did not have pediatric ARDS, n = 69; denied consent, n = 5). Thirty-two eligible, mechanically ventilated subjects meeting the PALICC diagnostic criteria (ARDS, n = 15; at risk for ARDS, n = 17) were finally enrolled in the study (Fig. 1). Of the 15 subjects with ARDS, 5 (33%) had mild ARDS and 10 (67%) had moderate ARDS. Subjects’ baseline data are shown in Table 1. At baseline, subjects with ARDS had higher OI and a lower ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen ( ), EELV, and CRS (Table 1).
), EELV, and CRS (Table 1).
Flow chart. NIV = noninvasive ventilation.
Subjects’ Demographic and Baseline Clinical Characteristics
Cumulative Relationships
A total of 896 measurements of lung mechanics were recorded in the 32 subjects. Correlation between EELV and CRS was significant in both studied groups (r = 0.60, P < .001). Figure 2 shows the positive correlations between static strain and EELV or CRS in the subjects with ARDS and in those at risk for ARDS. The same increases in EELV and CRS produced significantly more rapid increases of strain in the subjects with ARDS (ie, steeper coefficients) compared to the subjects at risk for ARDS (correlation coefficient differences between the 2 groups: z = –4.51 and z = –4.74, respectively; P < .01).
A: Correlations of EELV with strain in the ARDS group and the at risk group differed between the 2 groups, being steeper in subjects with ARDS (z = –4.51, P < .001). B: In contrast to the at risk group, CRS in subjects with ARDS did not correlate with the static strain. The correlations of CRS with strain also differed between the 2 groups, being steeper in subjects with ARDS (z = –4.74, P < .001). EELV and CRS were normalized to ΒΜΙ for comparison reasons. EELV = end-expiratory lung volume; CRS = respiratory system compliance; BMI = body mass index.
Longitudinal Lung Mechanics Responses to PEEP Changes
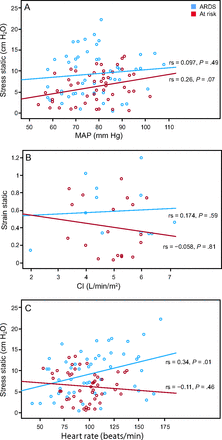
CRS was not affected by the PEEP levels in either group at day 1 (baseline) or at day 2 (48 h); EELV was higher with high PEEP, as expected, being comparatively higher in the group at risk for ARDS (Fig. 3). Strain and stress increased responding to increasing PEEP levels at baseline (P < .001) and at 48 h in subjects with ARDS (P = .037) and in subjects at risk for ARDS (P = .01). At all time points, strain and stress remained within safe limits in both groups (Fig. 4A–D). Data obtained in each measurement at each PEEP level in the 2 groups and their statistical differences are presented in the supplemental materials (see the supplementary materials at http://www.rcjournal.com). Neither strain nor stress in either group affected cardiac index or MAP at 10 cm H2O PEEP (Fig. 5). Heart rate increased with increasing stress (r = 0.34, P = .01) only in subjects with ARDS.
(A, B) CRS increasing trends between PEEP levels of 4 and 10 cm H2O did not reach statistical significance in the 2 pediatric ARDS groups at either day 1 (baseline) or day 2 (48 h). (C, D) EELV increased by increasing PEEP level from 4 and 10 cm H2O in the subjects with ARDS at all time points and in the subjects at risk for ARDS by 48 h. Boxes represent 25th and 75th percentiles, and center lines denote median. Whiskers show minimum and maximum values. Points represent outliers. EELV = end-expiratory lung volume; CRS = respiratory system compliance; BMI = body mass index.
Strain and stress increased responded to increasing PEEP levels from 4 to 10 cm H2O at baseline (P < .001) and 48 h in subjects with ARDS (P = .037) and those at risk for ARDS (P = .01). At all time points, strain and stress remained within safe limits in both groups. Boxes represent 25th and 75th percentiles, and center lines denote median. Whiskers show minimum and maximum values. Points represent outliers. Dashed lines indicate reported safety levels for strain and stress.
A: Scatterplots of cumulative correlations of stress with mean arterial pressure (MAP), (B) strain with cardiac index, and (C) stress with heart rate in the 2 studied groups of mechanically ventilated children at PEEP of 10 cm H2O. Fit lines at sub-cohorts are shown.
Longitudinal Similarities and Differences
The GLM/GEE model showed that ratios of CRS to body mass index (B = –0.62, 95% CI –0.7 to –0.54, P < .001) and EELV to body mass index (B = –21.6, 95% CI –24.3 to –18.8, P < .001) were significantly lower over time in the ARDS group compared to subjects at risk for ARDS. In addition, static strain (B = 0.15, 95% CI 0.1–2.0, P < .001) and static stress (B = 2.04, 95% CI 1.34–2.74, P < .001) became progressively higher in the ARDS group compared to subjects at risk for ARDS.
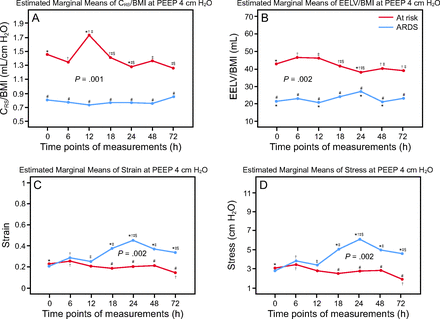
The longitudinal differences and trends were then studied at different PEEP levels and time points. At the low PEEP level (ie, 4 cm H2O), CRS (P = .001) and EELV (P = .002) were lower and strain and stress (P = .002) were higher in the subjects with ARDS compared to the subjects at risk for ARDS (Fig. 6). At the high PEEP level (ie, 10 cm H2O), CRS (P < .001) and EELV (P < .001) were also lower whereas strain and stress (P = .001) were significantly higher in the subjects with ARDS compared to those at risk for ARDS (Fig. 7). Throughout, from time point 0 (ie, baseline) to 72 h, CRS and EELV were steadily lower, and strain and stress were higher, in subjects with ARDS compared to subjects at risk for ARDS (Fig. 6, Fig. 7).
From time point 0 (baseline) to 72 h, CRS (P = .001), EELV (P = .002) were steadily lower and strain or stress (P = .002) were higher in subjects with ARDS compared to those at risk for ARDS at PEEP of 4 cm H2O (between-subject effects). # Indicates time point differences between subjects with pediatric ARDS and those at risk for ARDS. Multivariate simple effects of time within each level combination of pediatric ARDS groups showed: (A) CRS had interaction with the at risk group but not the ARDS group; (B) EELV had significant interaction with both groups; (C, D) strain and stress had significant interaction with the subjects with ARDS. Pairwise differences adjusted for within- and between-subject multiple comparisons (least significant differences): symbols with asterisks indicate time series significant differences (P < .05) within each group: *time point 0 (baseline) vs other time points; †6 h vs other time points; ‡12 h vs other time points; §18 h vs other time points (generalized linear model). EELV = end-expiratory lung volume; CRS = compliance; BMI = body mass index.
From time point 0 (baseline) to 72 h, CRS (P < .001) and EELV (P < .001) were steadily lower and strain and stress (P = .001) were higher in subjects with ARDS compared to those at risk for ARDS at PEEP of 10 cm H2O (between-subject effects). # Indicates time point differences between subjects with pediatric ARDS and those at risk for ARDS. Multivariate simple effects of time within each level combination of pediatric ARDS groups showed: (A) CRS had no interaction with either group; (B) EELV had significant interaction only with the group at risk for ARDS; (C, D) strain and stress had significant interaction with the subjects at risk for ARDS. Pairwise differences adjusted for within- and between-subject multiple comparisons (least significant differences): symbols with asterisks indicate time series significant differences (P < .05) within each group: *time point 0 (baseline) vs other time points; †6 h vs other time points; ‡12 h vs other time points; §24 h vs other time points (generalized linear model). EELV = end-expiratory lung volume; CRS = compliance; BMI = body mass index.
Multivariate Simple Effects of Time
We determined the multivariate effects of time in both pediatric ARDS groups at a PEEP of 4 cm H2O. For CRS, time interacted only with the subjects at risk for ARDS (subjects with ARDS: P = .73, effect = 0.15; subjects at risk for ARDS: P = .01, effect = 0.51, declining from 12 h to 72 h). For EELV, time interacted with both groups (subjects with ARDS: P = .03, effect = 0.46, increasing from 12 h to 24 h, declining from 24 h to 72 h; subjects at risk for ARDS: P = .04, effect = 0.44, declining from 12 h to 72 h). For strain, time interacted only with the ARDS group (subjects with ARDS: P = .02, effect = 0.49, increasing up to 24 h, decreasing from 24 h to 72 h; subjects at risk for ARDS: P = .09, effect = 0.38). For stress, time interacted only with the ARDS group (subjects with ARDS: P = .02, effect = 0.49, increasing up to 24 h, decreasing from 24 h to 72 h; subjects at risk for ARDS: P = .08, effect = 0.39). Pairwise comparisons adjusted for within- and between-subject multiple comparisons (least significant differences) are shown in Figure 6.
We also determined the multivariate effects of time in both pediatric ARDS groups at a PEEP of 10 cm H2O. For CRS, there was no time interaction with either group (subjects with ARDS: P = .19, effect = 0.31; subjects at risk for ARDS: P = .09, effect = 0.38). For EELV, time interacted only with the subjects at risk for ARDS (subjects with ARDS: P = .16, effect = 0.34; subjects at risk for ARDS: P = .01, effect = 0.51, declining from 6 h to 24 h). For strain, time interacted only with the subjects at-risk for ARDS (subjects with ARDS: P = .31, effect = 0.27; subjects at risk for ARDS: P = .001, effect = 0.66, declining from 6 h to 72 h). For stress, time interacted only with the subjects at risk for ARDS (subjects with ARDS: P = .22, effect = 0.30; subjects at risk for ARDS: P = .001, effect = 0.65, declining from 6 h to 72 h). Pairwise comparisons adjusted for within- and between-subject multiple comparisons (least significant differences) are shown in Figure 7.
Discussion
This study describes similarities and differences of lung mechanics between 2 pediatric ARDS groups using a noninvasive technique at the bedside. Our results indicate that similar increases of EELV or CRS in subjects with ARDS produce significantly higher strain and stress compared to the effects of increased EELV or CRS in subjects at risk for ARDS at PEEP levels up to 10 cm H2O. We also noted a weak increasing trend in CRS between PEEP levels of 4 and 10 cm H2O on the first and second days in the 2 study groups, whereas the EELV increase was pronounced in response to escalating PEEP levels, more consistent in the ARDS group. Strain and stress increased with increasing PEEP up to 10 cm H2O at all time points but remained within safe limits in both groups. Finally, we report that, during a 72-h period, CRS and EELV were steadily lower and strain and stress were higher in subjects with ARDS compared to subjects at risk for ARDS. Time–lung mechanics interactions also differed between the 2 groups at the studied PEEP levels.
Previous studies have reported that optimal values for CRS and ΔEELV/ΔPEEP could only be identified for each patient at PEEP levels of 10–17.5 cm H2O.22 This finding might explain why CRS remained substantially unchanged as PEEP increased up to 10 cm H2O in both study groups. In a recent study of mechanically ventilated pediatric subjects, the empirical probability of a positive response to PEEP increases was 67% for CRS.23 The unpredictable CRS response to escalated PEEP could be related to different structural characteristics among pediatric patients with various diseases characterized by inhomogeneity within the lungs.19 In contrast, EELV consistently increased in response to escalating PEEP levels in both groups from baseline to 72 h, confirming the results of a previous study showing that EELV did not change significantly for up to 7 d in subjects with ARDS.24 Similar to the greater EELV increases in response to incremental PEEP compared to the unpredictable CRS response to changes in PEEP shown in our series,14 the magnitude and range of values for ΔEELV/ΔPEEP were greater than those for CRS in an adult study targeting PEEP in subjects with ARDS.22 It has been argued that measuring ΔEELV/ΔPEEP might better detect recruitment and de-recruitment than measuring CRS because EELV alterations reflect steady-state changes in pressure and volume.22 Previous experimental25 and clinical studies26 have similarly questioned the reliability of CRS for lung recruitment/de-recruitment estimation in ARDS. It might be easier for the increased levels of PEEP to stretch the diaphragm through thoracic expansion during the initial (uncomplicated) hours, thus preventing the lung from being compressed by abdominal pressure.27 By counteracting the recoiling force of the lung to a lower volume, PEEP allows the chest wall to off-load from the lung and expand and suspend it further by displacing the diaphragm caudally.28 Accordingly, the method we used could be tried to measure recruitment through careful incremental titration of PEEP, which is now recommended for pediatric patients with ARDS rather than sustained inflation maneuvers.29 Furthermore, our results suggest that a PEEP rise sustains the initially achieved recruitment of the nondependent lung but does not recruit the dependent collapsed alveoli requiring high pressures each inspiratory cycle in both patients with ARDS and in patients at risk for ARDS.28,30
Blankman et al20 reported that stress and strain can be calculated reliably at the bedside based on noninvasive EELV measurements during a decremental PEEP trial involving pressure control ventilation of 26 adults with coronary artery bypass graft or neurological or lung disorders. The large variation in respiratory mechanical properties among children demands that ventilator settings be titrated to lung and chest wall mechanics.31 For the first time, using multivariate simple effects of time pediatric subjects with ARDS and pediatric subjects at risk for ARDS, we noted important lung mechanic similarities and differences between these subjects. Because the injured lung is not uniformly expanded, pleural pressure and stress are not uniformly distributed, resulting in stress concentrations between more or less distorted heterogeneous regions.32 The steadily increased strain and stress related to ARDS in this study assumes that this phenomenon is more exacerbated in subjects with ARDS compared to subjects at risk for ARDS, expanding results of previous studies that reported higher strain and stress in adults and in children with lung injury compared to healthy individuals.14,27
The “lung injury” limits for strain may be exceeded by increasing the VT in patients with low CRS. Depending on recruitment potential, static hyperaeration produced by unnecessarily high PEEP might add tidal (dynamic) volume to the static hyperinflation.18 In our study, by keeping VT low, static strain did not exceed 1.5 and stress did not exceed 20 cm H2O at PEEP of 10 cm H2O33 and did not interfere with hemodynamics at 10 cm H2O PEEP in either group. In an animal model, dynamic strains > 2.0 caused pulmonary edema and death within 54 h.34 Pelosi et al35 recently proposed that permissive atelectasis might be at least as effective as the open lung strategy with the advantage of minimizing overstretch of the lung parenchyma and mitigating hemodynamic consequences. Recent clinical and experimental studies have reported stress values of 19–21.8 and 11–13.3 cm H2O at 15 and 5 cm H2O of PEEP, respectively, at VT of 8–10 mL/kg4,19; other studies have reported that applied strain > 2 may be injurious or lethal for the lung.20,34 In a respective study involving mechanically ventilated children with ARDS, stress values were 16–27 cm H2O at 4 and 12 cm H2O of PEEP at VT of 8–12 mL/kg.36
Although our study groups were small, limiting the study’s power and ability to reach definitive conclusions, this study size is powered for the 896 measurements done and compares favorably to the sample sizes of similar studies.22,25,26,34,36 Also, the validity of calculation equations or k values used has never been adequately assessed in critically ill children. The technique used in its current format did not allow us to include subjects supported with  > 0.60, thereby excluding patients with severe ARDS. Updated modules, however, would allow measurements with
> 0.60, thereby excluding patients with severe ARDS. Updated modules, however, would allow measurements with  up to 0.85, extending its use to this group of subjects. No esophageal pressure measurements were performed to assess lung mechanics in our subjects, allowing measurement of elastance and comparison of the 2 methods in a validation study. It has been reported, however, that esophageal balloon inflation ranges do not assure accuracy, suggesting that better esophageal catheters are needed to provide reliable esophageal pressure measurements in children.37 Obviously, a validated method for measuring lung mechanics in children lags behind. We have now set up a pilot comparative study of esophageal and noninvasive measurements in subjects with and without pediatric ARDS. Comparative studies with upgraded techniques are needed to ascertain whether this noninvasive strategy could help optimize our decisions on setting and longitudinally modifying ventilator parameters in individual subjects.
up to 0.85, extending its use to this group of subjects. No esophageal pressure measurements were performed to assess lung mechanics in our subjects, allowing measurement of elastance and comparison of the 2 methods in a validation study. It has been reported, however, that esophageal balloon inflation ranges do not assure accuracy, suggesting that better esophageal catheters are needed to provide reliable esophageal pressure measurements in children.37 Obviously, a validated method for measuring lung mechanics in children lags behind. We have now set up a pilot comparative study of esophageal and noninvasive measurements in subjects with and without pediatric ARDS. Comparative studies with upgraded techniques are needed to ascertain whether this noninvasive strategy could help optimize our decisions on setting and longitudinally modifying ventilator parameters in individual subjects.
Conclusions
This study, which is the first attempt to compare group responses to PEEP over time, indicates that CRS and EELV were lower, strain and stress were higher, and their interrelations were weaker in subjects with ARDS compared to subjects at risk for ARDS during a 72-h period. Using a noninvasive nitrogen washout/washin technique, we noted that these parameters behaved differently over time at PEEP values of 4 or 10 cm H2O and that, at all time points, strain and stress remained within safe limits in both groups. Further studies are needed to validate these findings and to better describe the 2 pediatric ARDS phenotypes, optimizing lung mechanics while reassuring longitudinal lung-protective ventilation in individual subjects.
Footnotes
- Correspondence: George Briassoulis MD PhD, Pediatric Intensive Care Unit, University Hospital of Heraklion Medical School, University of Crete, 71110 Heraklion, Crete, Greece. E-mail: ggbriass{at}otenet.gr
The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2021 by Daedalus Enterprises