Abstract
BACKGROUND: High-dose (≥ 80 ppm) inhaled nitric oxide (INO) has antimicrobial effects. We designed a trial to test the preventive effects of high-dose NO on coronavirus disease 2019 (COVID-19) in health care providers working with patients with COVID-19. The study was interrupted prematurely due to the introduction of COVID-19 vaccines for health care professionals. We thereby present data on safety and feasibility of breathing 160 ppm NO using 2 different NO sources, namely pressurized nitrogen/NO cylinders (INO) and electric NO (eNO) generators.
METHODS: NO gas was inhaled at 160 ppm in air for 15 min twice daily, before and after each work shift, over 14 d by health care providers (NCT04312243). During NO administration, vital signs were continuously monitored. Safety was assessed by measuring transcutaneous methemoglobinemia (SpMet) and the inhaled nitrogen dioxide (NO2) concentration.
RESULTS: Twelve healthy health care professionals received a collective total of 185 administrations of high-dose NO (160 ppm) for 15 min twice daily. One-hundred and seventy-one doses were delivered by INO and 14 doses by eNO. During NO administration, SpMet increased similarly in both groups (P = .82). Methemoglobin decreased in all subjects at 5 min after discontinuing NO administration. Inhaled NO2 concentrations remained between 0.70 ppm (0.63–0.79) and 0.75 ppm (0.67–0.83) in the INO group and between 0.74 ppm (0.68–0.78) and 0.88 ppm (0.70–0.93) in the eNO group. During NO administration, peripheral oxygen saturation and heart rate did not change. No adverse events occurred.
CONCLUSIONS: This pilot study testing high-dose INO (160 ppm) for 15 min twice daily using eNO seems feasible and similarly safe when compared with INO.
- nitrogen dioxide
- electric NO generator
- nitric oxide
- methemoglobin
- spontaneous breathing
- pulmonary vasodilator
Introduction
Nitric oxide (NO) gas is approved by the United States Food and Drug Administration for the treatment of hypoxia associated with pulmonary hypertension in the newborn.1,2 In clinical practice, NO gas is widely used to reduce pulmonary artery pressure and to improve oxygenation in adult patients with ARDS.3-5 An elevated dose of NO gas at 160 ppm and higher has previously been proposed to produced antibacterial and antiviral activity in experimental studies6,7 as well as pediatric and adult patients.8–10 Recent in vitro studies demonstrate that NO donors can inhibit replication of the SARS-CoV-2 virus,11 and clinical trials are investigating the clinical benefits of high-dose inhaled NO (INO) in patients with coronavirus disease 2019 (COVID-19).
NO gas is commonly administered with delivery systems that use pressurized NO in nitrogen (NO/N2) cylinders. Pressurized INO cylinders are widely available and have been used in more than a half million patients worldwide.12 Despite being safe and reliable, the use of pressurized cylinders as the source of NO requires an extensive supply chain and trained personnel to deliver and manage the (NO/N2) cylinders. Further, cylinder NO therapy can be expensive.13
Electrical NO (eNO) generators have been proposed as an alternative source. These devices ionize air (nitrogen and oxygen) with a pulsed, high-voltage electrical discharge leading to the generation of NO, nitrogen dioxide (NO2), and metal microparticles (released by the electrodes during electrical discharge).14,15 A scavenger containing calcium hydroxide can reduce NO2 levels below the safety threshold (< 3 ppm for NO2),16 whereas a 0.22-micron high-efficiency particulate air (HEPA) filter removes metal particles generated by electric discharge.17 These eNO devices can provide INO therapy without the need for bulky and expensive cylinders, potentially making NO therapy widely available both inside and outside the hospital.
To evaluate the preventive effects of NO in COVID-19, a clinical trial of health care workers was performed. Subjects were randomized to the treatment group (subjects received NO via INO or eNO according to what was available) or to the control group (subjects did not receive any gas). This analysis aimed to evaluate the feasibility and safety of administering 160 ppm to spontaneously breathing healthy volunteers using INO and eNO in the treatment group.
QUICK LOOK
Current Knowledge
The clinical application of high-dose nitric oxide (NO) to treat bacterial and viral infections is under investigation through several clinical trials. The standard NO source is a pressurized cylinder containing NO balanced in nitrogen. Over the last several years, electric NO (eNO) generators capable of generating NO from air using a pulsed electrical discharge have been developed.
What This Paper Contributes to Our Knowledge
High-dose NO was successfully administered to healthy volunteers using both pressurized cylinders and eNO generators as an NO source. The delivery of high-dose NO in this pilot study appeared to be feasible and safe using both sources. Methemoglobin increased in the same fashion in both groups. The delivered nitrogen dioxide levels remained below prescribed safety levels.
Methods
This analysis uses data from the trial of health care workers who were enrolled between March 2020 and August 2020 (NCT04312243). This study was reviewed and approved by the institutional review board at Massachusetts General Hospital (protocol 2020P000831). Written informed consent was obtained from each subject prior to initiation of any study procedures. The trial was terminated early (March 10, 2021) due to a lack of enrollment after the approval of COVID-19 vaccines. Data from the enrolled participants who received NO were assessed for safety and feasibility of administration.
Subject Selection
Enrolled subjects were adult (≥ 18 y) health care workers (physicians, nurses, or respiratory therapists) working at Massachusetts General Hospital who were scheduled to work with SARS-CoV-2-positive patients at least 3 times a week (defined as 6 or more shifts in 14 d). Individuals were excluded if they previously had a positive SARS-CoV-2 reverse transcription-polymerase chain reaction test, were pregnant, or had a history of hemoglobinopathies or anemia.
Nitric Oxide Gas Administration
The study subjects received NO at 160 ppm for 15 min twice per day, before and after each work shift, over 14 d. The antiviral and antibacterial NO dose, the duration of a single treatment, and the number of treatments are now under investigation. Some authors suggest few breaths or short intermittent pulses of high NO concentrations might work better than continuous inhalation of standard dose of NO.7 However, it requires thorough experimental investigation and clinical studies. In our laboratory, we performed a study testing high concentration of NO in a murine model of Klebsiella pneumonia.6 We found that short periods of breathing high concentration of NO (12 min of NO at 300 ppm every 3 h) were more efficient in eliminating Klebsiella pneumonia than continuous NO breathing at lower concentrations (80 ppm, 160 ppm, or 200 ppm for 48 h). It needs to be determined the effective antiviral and anti-SARS-CoV-2 concentrations, the duration of administration, and the number of administrations required.
We decided to give 160 ppm as previously reported to be safe in humans. We used 15 min to mimic our experimental study in mice. We decided to administer NO before and after the work shift.
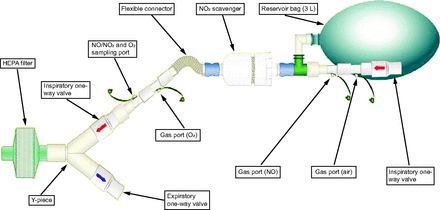
To provide high concentrations of NO breathing, a face mask and apparatus that were previously designed and tested were utilized.18 Briefly, the apparatus is composed of standard respiratory circuit connectors, a 3-L reservoir bag, a scavenger containing powdered calcium hydroxide, a 0.22-micron HEPA filter, and a snug-fitting mask (Fig. 1). Since high-dose NO reacts with the circulating hemoglobin producing methemoglobin, transcutaneous methemoglobin (rainbow SET, Masimo, Irvine, California) was monitored. Using the same device, 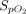 and heart rate were evaluated. Heart rate,
and heart rate were evaluated. Heart rate, 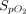 , and methemoglobinemia (SpMet) data were collected before and at the end of NO administration. To continuously monitor
, and methemoglobinemia (SpMet) data were collected before and at the end of NO administration. To continuously monitor 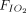 , an oxygen analyzer (MiniOX 1, Ohio Medical, Gurnee, Illinois) was used.
, an oxygen analyzer (MiniOX 1, Ohio Medical, Gurnee, Illinois) was used.
Apparatus used to deliver high-dose inhaled nitric oxide. NO = nitric oxide, HEPA = high-efficiency particulate air filter.
NO and NO2 Monitoring
To avoid variation of NO gas concentration during the respiratory cycle, the reservoir gas flow was kept constant at 15 L/min during NO treatments.18 Levels of NO and NO2 were monitored through a sampling line connected to the inspiratory limb of the circuit, proximal to the patient. NO was measured by chemiluminescence (Sievers 280i Nitric Oxide Analyzer, GE Analytical Instruments, Boulder, Colorado), and cavity-attenuated phase shift (Aerodyne Research, Billerica, Massachusetts) was used to monitor NO2 levels. When NO and NO2 concentrations using high-sensitivity methods (chemiluminescence and cavity-attenuated phase shift) were not available, we set NO and O2 flow as shown in Table S1 (see related supplementary material at available at http://rc.rcjournal.com). In subjects breathing from eNO generator, the delivered NO-NO2 concentrations were always measured. To determine NO absorption and NO2 production in the airway, exhaled NO and NO2 concentrations were measured in one healthy subject during eNO administration.
eNO and INO
Two different NO sources were studied, including a pressurized cylinder containing 850 ppm of NO/N2 (150 A, Airgas, Radnor Township, Pennsylvania) (content = 4,089 L at STP) and an eNO generator. The eNO generator (portable NO generator, Odic. Littleton, Massachusetts) combines a gas pump, an NO generation chamber containing an iridium spark plug, an 18-g scavenger containing calcium hydroxide, and a 0.22-µm HEPA filter.17 To obtain the desired NO concentration, the generator was set with the following sparking parameters: sparking frequency 85 Hz and duty cycle 65%, with an air flow of 2.5 L/min. The decision to use eNO or INO was based on subject preference, material, or personnel availability. Any subject could receive NO using either NO source (INO and eNO).
Statistical Analysis
Data are reported as mean (SD) or median (interquartile range [IQR]) for continuous variables and as frequencies and proportions for categorical variables. Normality was assessed using the Shapiro-Wilk test. To evaluate the trend of a continuous variable over time (before and after the treatment), a mixed effect model (R package [lme4] counting each patient as a random effect, R package [emmeans] for post hoc analysis) was used. Statistical significance was determined as a 2-tailed P < .05. All the analyses were conducted using R Core Team (2021) (R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Population
We enrolled a total of 24 subjects: 12 in the treatment group and 12 in the control group. None of the enrolled subjects developed COVID-19. The control group (subjects not receiving NO) was not included in the presented analysis.
NO was administered to 12 subjects, including 6 males and 6 females. Overall, the mean (SD) age was 43.3 y (12.70) with a body mass index of 28.9 kg/m2 (5.57). Two subjects had a past medical history of systemic hypertension and type 2 diabetes mellitus, and one received chronic bronchodilator therapy for asthma. Study population description is presented in Table S2(see related supplementary material at available at http://rc.rcjournal.com).
Nitric Oxide Administrations
Twelve subjects received a total of 185 NO gas administrations. INO was used to administer 171 doses, and eNO was used for 14 doses. Each subject received, on average, 15.4 NO administrations. All the study subjects received NO using the INO source. Three subjects received NO using both INO and eNO.
Air flow was maintained at 15 L/min in the reservoir for all treatments. An NO flow of 4 (0) L/min at 850 ppm in nitrogen was added when using pressurized cylinders and 2.5 (0) L/min at ∼ 1,180 ppm in air when using the eNO generator. 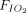 was 0.21 in all administrations. When an NO/N2 pressurized cylinder was used, 1 L/min of supplemental oxygen was added to maintain the
was 0.21 in all administrations. When an NO/N2 pressurized cylinder was used, 1 L/min of supplemental oxygen was added to maintain the 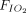 at 0.21. If the minute ventilation is higher than the delivered total flow (air flow + O2 flow + NO flow), room air will enter the delivery system from an inspiratory valve on the inspiratory limb of the delivery system (Fig. 1).
at 0.21. If the minute ventilation is higher than the delivered total flow (air flow + O2 flow + NO flow), room air will enter the delivery system from an inspiratory valve on the inspiratory limb of the delivery system (Fig. 1).
Methemoglobin
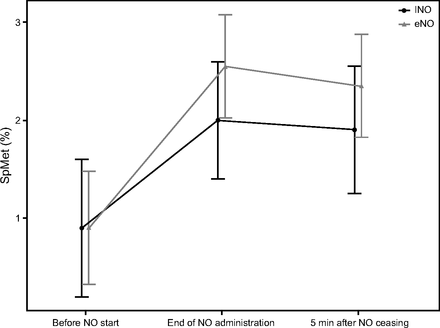
During the study gas administration, SpMet increased in both groups from 0.90% (0.10) to 1.98% (0.11) with INO (95% CI −1.44 to −70, P < .001) and from 0.85% (0.17) to 1.89% (0.16) with eNO (95% CI −1.64 to −0.42, P < .001). The increase in SpMet was not statistically different between eNO and INO administrations (95% CI −0.29 to 0.46), P = .98; Fig. 2). Five minutes after stopping NO administration, SpMet decreased to 1.87% (0.11) (95% CI −0.01 to 0.22, P = .09) and 1.81% (0.16) (95% CI −0.32 to 0.48, P = .94) with INO and eNO, respectively.
Noninvasive peripheral saturation of methemoglobin (SpMet) before initiating nitric oxide (NO) gas at the end of NO administration and 5 min after cessation. INO = pressurized NO/N2 cylinder; eNO = electric NO generator.
NO and NO2 Delivered Concentrations
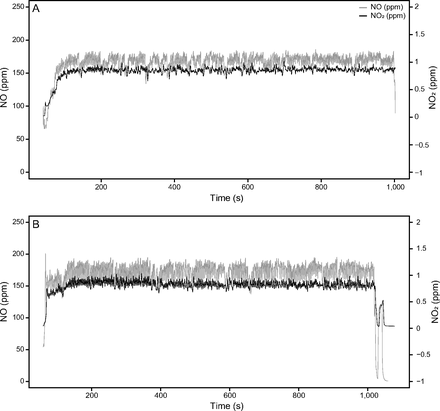
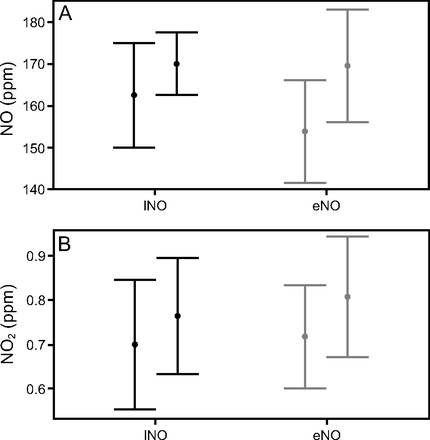
INO and NO2 concentrations were continuously monitored over 54 administrations, including 39 with INO and 14 with eNO (Fig. 3). During the study, NO concentration median ranged between 164 ppm (156–169) and 170 ppm (165–175) with INO and between 153 ppm (151–163) and 178 ppm (158–180) with eNO. NO2 concentrations varied between 0.70 ppm (0.63–0.79) and 0.75 ppm (0.67–0.83) with INO and between 0.74 ppm (0.68–0.78) and 0.88 ppm (0.70–0.93) with eNO (Fig. 4). There was no statistically significant difference between the delivered NO2 concentrations between INO and eNO (95% CI 0.04–0.17, P = .35). As shown, the intratidal variations of NO concentration are significantly higher with eNO 14.85 ppm (10.60) compared to INO 8.53 ppm (2.90) (95% CI −12.30 to −0.35, P = .038). This difference may be explained by the higher total gas flow (air flow + NO flow + oxygen flow) with the INO source 20.0 L/min as compared to eNO 17.5 L/min.
Nitric oxide (NO) and nitrogen dioxide (NO2) concentration during the 15-min study gas administrations. Panel A depicts the use of a pressurized cylinder (INO) as an NO source. In Panel B, the gas source was the electric NO (eNO) generator.
Nitric oxide (NO) (Panel A) and nitrogen dioxide (NO2) (Panel B) intratidal concentration variation (minimum on the left and maximum on the right for each NO source) with INO and eNO. INO = pressurized NO/N2 cylinder; eNO = electric NO generator.
Exhaled NO and NO2
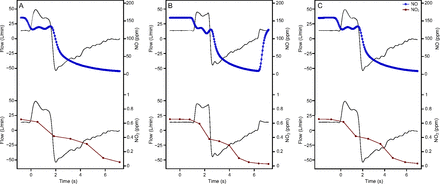
To further capture safety data, exhaled NO and NO2 were measured in one health care worker receiving eNO. The average inspired NO and NO2 concentrations were 158.5 ppm and 0.7 ppm, respectively. At the end of exhalation (alveolar gas phase), NO concentration decreased to 11.8 ppm, and NO2 concentration was 0.027 ppm (Fig. 5), suggesting minimal NO2 generation in the airways.
Inspiratory and expiratory nitric oxide (NO) (blue) and nitrogen dioxide (NO2) (red) concentrations during 3 consecutive breaths (Panel A, B, C).
Vital Signs During NO Administration
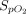 decreased slightly from 97% (97–98) before NO administration to 96% (95–97) at the end of NO administration (95% CI 1.5–2.0, P < .001) with INO. When eNO was used,
decreased slightly from 97% (97–98) before NO administration to 96% (95–97) at the end of NO administration (95% CI 1.5–2.0, P < .001) with INO. When eNO was used, 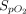 remained unchanged (P = .57).
remained unchanged (P = .57).
Heart rate was slightly reduced from 80.0 beats/min (72.0–87.0) to 78.0 beats/min (70.5–85.0) (95% CI 2.0–3.5, P < .001) with INO and from 79.0 beats/min (73.0–82.0) to 74.5 beats/min (70.0–77.0) (95% CI 2.5–8.0, P < .001) with eNO; (Fig. 6) During the administrations, none of the study subjects reported any discomfort. None of the subjects developed symptoms such as cough and wheezing, suggesting a not significant generation of nitric acid into the airways (from the reaction between NO2 and bronchial moisture). No adverse events were noted.
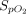 (Panel A) and heart rate (Panel B) before nitric oxide (NO) gas administration and at the end of NO administration. INO = pressurized NO/N2 cylinder; eNO = electric NO generator.
(Panel A) and heart rate (Panel B) before nitric oxide (NO) gas administration and at the end of NO administration. INO = pressurized NO/N2 cylinder; eNO = electric NO generator.
Discussion
Over 185 consecutive NO administrations, we showed that administering high-dose NO (160 ppm) for 15 min using an eNO generator appears to be feasible and as safe compared to NO delivered from pressurized cylinder-based delivery systems. We were able to reach and maintain a stable NO concentration of 160 ppm with both INO and eNO throughout the 15-min administration interval. All NO administrations were well tolerated and without any adverse events. Volunteers were comfortable as suggested by their significant reductions of heart rate during the administrations.
During the NO administrations, SpMet rose in a similar fashion (with a percentage increase of 55–64%) with both NO sources, suggesting a similar biological effect. Five min after the end of the administration, SpMet decreased in both groups, reflecting a robust reduction of methemoglobin by the subjects’ methemoglobin reductase.
Monitoring NO2 concentration is imperative when administering NO at high levels. Inhaled NO2 concentration, despite being slightly higher with eNO (but not statistically significant), was below the Occupational Safety and Health Administration safety levels. Although not measured during these administrations, we reported low levels of ozone produced by the eNO generator in previous studies.19
The administration of high-dose INO led to encouraging results in a patient with cystic fibrosis and chronic lung infection10 and improved oxygenation and reduced hospital stay in 69 infants admitted with acute bronchiolitis.9 During the COVID-19 pandemic, 6 pregnant women with severe or critical COVID-19 pneumonia were treated with high-dose NO (160–200 ppm) for 30 minutes twice a day, resulting in improved oxygenation and a reduction in breathing frequency. INO produced symptom relief of shortness of breath and dyspnea in these patients.20
The main limitation of the widespread use of high-dose INO is the need for dedicated personnel to manage bulky equipment and cylinders. The eNO generator we studied weighs 1.5 kg, reducing the need for trained personnel or bulky materials, making it easy to use during a pandemic or in a low-resource setting. The development of novel eNO generators delivering high-dose NO from air that continuously monitor inhaled NO/NO2 concentration and transcutaneous methemoglobin will facilitate the use of eNO for ambulatory and home use (particularly important for remote or low-resource areas).
The main limitation of this study is that the decision to use INO or eNO was based on availability of materials (tanks and eNO generator) and preference of the subjects rather than random assignment. Thus, the number of high-dose NO administrations using INO and eNO was unequal. Lastly, one should note that the number of administrations using eNO in the presented case series is limited.
Conclusions
This is the first pilot study showing promising preliminary results on feasibility and safety of high-dose NO (160 ppm) using an eNO generator. All volunteers tolerated well the treatments, and the increase in SpMet during NO gas delivery is comparable between INO and eNO.
Footnotes
- Correspondence: Lorenzo Berra MD, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, Massachusetts 02141. E-mail: lberra{at}mgh.harvard.edu
This study was registered on clinicaltrials.gov (NCT0431224).
Supplementary material related to this paper is available at http://rc.rcjournal.com.
Drs Zapol and Yu disclose a relationship with Third Pole. Dr Carroll discloses a relationship with UNITAID. Dr Berra discloses relationships with iNO Therapeutics, Praxair, Masimo, the National Institutes of Health, and Fast Grant. The remaining authors have disclosed no conflicts of interest.
 noninvasive monitoring devices were offered at no cost by Masimo. The study was supported by departmental funds.
noninvasive monitoring devices were offered at no cost by Masimo. The study was supported by departmental funds.↵† Deceased.
- Copyright © 2022 by Daedalus Enterprises