Abstract
BACKGROUND: The Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) is widely employed in assessing functional decline in individuals with amyotrophic lateral sclerosis (ALS). A limitation of the scale is that item 12 does not directly evaluate worsening respiratory failure in ALS but rather the management thereof as a surrogate marker. We propose an alternative scale to assess respiratory function in ALS individuals who do not use noninvasive ventilation (NIV).
METHODS: 85 participants were included in the study. ALSFRS-R scores were calculated and FVC measured at each clinic visit. Additional questions were asked regarding the presence of nocturnal hypoventilation symptoms, including (1) early-morning headaches, (2) excessive daytime somnolence, (3) poor concentration, and (4) decrease in appetite. A nocturnal hypoventilation item was developed using these questions in participants not using NIV. Internal consistency and validity were calculated using the nocturnal hypoventilation item as substitute for the existing item 12. The ALSFRS-R was modified by adding the alternative item 12 and named ALSFRS-Revised Modified (ALSFRS-RM).
RESULTS: The ALSFRS-RM has a strong internal consistency and validity, which was calculated using Cronbach alpha and factor analysis. A Spearman correlation of 0.34 was calculated between the measured FVC and the nocturnal hypoventilation item score. In addition, a nocturnal hypoventilation item score of ≤ 3 corresponds to an FVC of ≤ 65%, with the upper 95% CI < 80%.
CONCLUSIONS: Our results suggest that the addition of an alternative item 12 to the existing ALSFRS-R may be a viable option for use in individuals not receiving ventilatory support. The new nocturnal hypoventilation item may also be a reliable indicator of respiratory decline that may remove the need for FVC measurement prior to introducing NIV.
- ALSFRS-R
- amyotrophic lateral sclerosis
- motor neuron disease
- functional scale
- respiratory failure
- noninvasive ventilation
- vital capacity
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder that leads to weakness of skeletal muscles, including those responsible for respiratory function. Due to sleep-associated loss of tone in rib cage muscles, more reliance on diaphragmatic muscles is required at nighttime. The muscles of the diaphragm inevitably become weak in individuals with ALS, which in turn leads to nocturnal hypoventilation. As nighttime blood oxygen content declines and carbon dioxide levels rise, nocturnal hypoventilation symptoms emerge. These symptoms include frequent awakenings, excessive daytime somnolence, early-morning headaches, impaired concentration, and poor appetite.1 Noninvasive ventilation (NIV) is the standard treatment used to alleviate these symptoms, but access to this treatment modality is not universal. Furthermore, not all individuals tolerate NIV; and factors such as discomfort or significant bulbar weakness may limit its use, whereas others may choose not to use NIV for personal or cultural reasons.2
The Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) and its revision (ALSFRS-R) are disease-specific, questionnaire-based scales employed in assessing the functional status of individuals with ALS. As a measurement tool, it has a strong internal consistency and validity3-5 and is widely used in both clinical practice and trials due to its simplicity and cost-effectiveness. In addition, the questionnaire can be administered remotely as it is a patient-reported outcome tool and no clinical examination or special equipment is required.5-7 The ALSFRS can be further subdivided into subscores for 4 different body regions, namely bulbar (ALSFRS-b), upper limb (ALSFRS-ul), lower limb (ALSFRS-ll), and respiratory function (ALSFRS-rsp). Whereas the ALSFRS included a single item on respiratory function, the revised version of the scale (ALSFRS-R)3 introduced separate assessments of dyspnea (item 10), orthopnea (item 11), and the use of NIV (item 12). Consequently, equal weight is placed on respiratory function as compared to the limb and bulbar components.
However, a potential problem arises with the application of the ALSFRS-R in individuals with respiratory insufficiency who do not use NIV. Item 12 measures the quantity of NIV use by the individual during the day and night. Therefore, item 12 does not directly evaluate worsening respiratory symptoms in ALS but rather the management thereof as a surrogate marker. This is problematic, as the item fails to reflect the true respiratory status of individuals with ALS with respiratory involvement who are not using NIV.
Furthermore, there appears to be a poor correlation between the respiratory subscore (ALSFRS-R-rsp) and both objective respiratory measures (such as vital capacity) and disease progression, with improvement in the subscore even documented in some individuals.8 Finally, the application of item 12 ultimately assumes access to objective respiratory-function measurement by means of specialized equipment, which is required prior to starting NIV. This is likely to influence both the extent and timing of NIV introduction, especially in limited-resource settings.
The above limitations highlight the need to further refine the ALSFRS-R, in particular item 12. In an attempt to do so, we investigated the utility of replacing the existing item 12 (use of NIV) with a question directly assessing symptoms of nocturnal hypoventilation, intended for use in individuals not utilizing NIV. In addition, we hypothesized that this substitution would provide similar or better internal consistency and correlation with the percentage of predicted FVC (FVC%) than the current item 12 of the ALSFRS-R. Nocturnal hypoventilation is prevalent in chronic ventilatory disorders and probably precedes daytime respiratory failure.9,10 Although item 11 evaluates orthopnea, it could be argued that this is a symptom of positional and not necessarily nocturnal hypoventilation. Clearly orthopnea contributes to nocturnal hypoventilation, but other factors like sleep-disordered breathing are also involved in the development of symptoms. Including an item assessing symptoms of nocturnal hypoventilation would, therefore, theoretically increase the utility of the ALSFRS-R in detecting and quantifying respiratory weakness.
QUICK LOOK
Current Knowledge
The Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) is widely employed in assessing functional decline in individuals with amyotrophic lateral sclerosis (ALS). A limitation of the scale is that item 12 does not directly evaluate worsening respiratory failure in ALS but rather the management thereof as a surrogate marker.
What This Paper Contributes to Our Knowledge
Our findings suggest that the addition of an alternative item 12 to the existing ALSFRS-R may be a viable option to assess respiratory function in individuals with ALS who are not using noninvasive ventilation (NIV). The new nocturnal hypoventilation item also appears to be a potential indicator of significant respiratory dysfunction, which may remove the need for FVC measurement prior to introducing NIV.
Methods
Participants, Data Collection, and Study Design
We included all participants, ≥ 15 y of age, who were diagnosed with ALS (according to the revised El Escorial/Airlie House criteria11) or progressive muscular atrophy (PMA) over a 4-y period between July 1, 2014–June 30, 2018. All participants were diagnosed and followed up at the multidisciplinary ALS clinic in the Division of Neurology, Tygerberg Academic Hospital, Cape Town, South Africa. We only included participants who had attended the clinic at least twice at the time of analysis. Data were collected prospectively as part of an epidemiological study and managed using REDCap electronic data capture tools hosted at Stellenbosch University.12,13 Study approval was obtained from the Human Research Ethics Committee of Stellenbosch University (Ethics reference no.: N13/06/085). All participants provided informed consent.
At each clinic visit, routine assessment included the ALSFRS-R and seated FVC measurement. FVC was measured using a CareFusion Micro 1 Handheld Spirometer (Vyaire Medical, Mettawa, Illinois). For each FVC measurement, an FVC% was calculated by comparing it to projected values for age, gender, and height.14
In addition, a set of questions assessing nocturnal hypoventilation was administered, each requiring a “yes” or “no” response. These questions evaluated (1) early-morning headaches, (2) excessive daytime somnolence, (3) poor concentration, and (4) decrease in appetite. These symptoms are regarded as indicators of respiratory insufficiency in ALS, along with dyspnea, orthopnea, apathy, difficulty clearing secretions, and disturbed sleep not due to pain.10,15-17 The last 5 symptoms were excluded from the questionnaire, dyspnea and orthopnea because they are already incorporated into the ALSFRS-R (items 10 and 11), apathy and difficulty clearing secretions because of their nonspecific nature, and disturbed sleep due to concern of a possible overlap with orthopnea. These questions were collectively named “nocturnal hypoventilation symptoms” and were weighted equally. The number of “yes” responses were subtracted from 4, which produced a nocturnal hypoventilation item score, with zero indicating the most severe nocturnal hypoventilation symptoms and 4 no symptoms. Although the other items of the ALSFRS-R are ordinal in nature (including the existing item 12), we could find no literature to support a specific sequence of appearance of these symptoms as nocturnal hypoventilation worsens. Therefore, for the purpose of this exploratory study, we constructed the alternative item 12 as a summated score; that is, we assumed severity is related to the number of symptoms and not their sequence of appearance. The nocturnal hypoventilation item was retrospectively incorporated into the existing ALSFRS-R as an alternative item 12 and designated 12a (intended for use in individuals not utilizing NIV), whereas the original item 12 was designated 12b (intended for use in individuals utilizing NIV) (Table 1). For ease of reference, we refer to this modified scale as ALSFRS-Revised Modified (ALSFRS-RM).
Item 12 of the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised Modified Incorporating a Proposed Alternative Item 12 for Patients not Using Noninvasive Ventilation (12a) and the Original Item 12 for Patients Using Noninvasive Ventilation (12b)1
Statistical Analysis
Each visit was regarded as an individual data point. Only data points recorded before introduction of NIV were included in the analysis, as our stated aim was specifically to assess the utility of a modified item 12 in individuals not using NIV. Furthermore, nocturnal hypoventilation symptoms are likely to be at least partially relieved by ventilatory support (which is in part the purpose of NIV), despite ongoing disease progression. In order to accurately assess correlation between item 12 and the FVC%, we also excluded data points where no FVC value was available. The first data point for each participant was collected at the time of diagnosis and enrollment into the study. Subsequent data points were collected at individual follow-up clinic visits.
For all parameters, mean ± SD, mean with 95% CI. The internal consistency of the ALSFRS-RM scale was assessed by calculating Cronbach alpha and factor analysis with varimax rotation. The strength of the correlation between item 12a and FVC% was evaluated using Spearman correlation coefficient. Similar FVC% cross-sectional correlation analyses were calculated for each ALSFRS-RM subscore (ALSFRS-RM-b, ALSFRS-RM-ul, ALSFRS-RM-ll, and ALSFRS-RM-rsp) as well as for the total ALSFRS-RM and ALSFRS-R scores using Spearman correlation coefficient. Differences in means between groups were determined using one-way ANOVA with the Bonferroni correction for multiple comparisons. Statistical analysis was performed using Stata statistical software (Release 13, StataCorp, College Station, Texas). Statistical significance was set at P < .05.
Results
A total of 85 participants were included, 49 of whom were male, with a mean age of 60 (30–86). Mean duration of illness at inclusion was 13 months (1–60). Seventy-one participants had ALS, whereas the remaining 14 had PMA. The demographic and baseline characteristics are presented in Table 2. A total of 182 data points were available for analysis (Supplementary Table 1, see related supplementary materials at http://www.rc.rcjournal.com). There were 56 datapoints (31%) where item 12a identified respiratory involvement not identified by the existing item 12. In 99 data points with a normal ALSFRS-R-rsp subscore (12/12), the ALSFRS-RM-rsp subscore was abnormal in 13, thereby identifying an additional number of participants with symptoms of respiratory dysfunction.
Demographics and Characteristics of Included Subjects
Factor Analysis
The internal structure of the ALSFRS-RM score was evaluated using factor analysis. The results (Table 3) revealed that evaluation items cluster into 3 factors that account for 74.5% of the total variance. The 3 factors correspond to upper- and lower-limb functioning (factor 1), respiratory functioning (factor 2), and bulbar functioning (factor 3). Although this is similar to previously reported divisions of the ALSFRS-R scale, it is slightly different in that upper- and lower-limb functioning falls within the same factor.3 However, the new nocturnal hypoventilation item groups together with the existing respiratory-functioning questions (dyspnea and orthopnea), which implies that its addition leaves the remainder of the scale unaltered.
Rotated Factor Loadings
Internal Consistency
Table 4 displays the Cronbach alpha of the ALSFRS-RM index (raw and standardized values). Both the raw and standardized scale are internally consistent, with alphas exceeding 0.82. The raw and standardized Cronbach alphas for the ALSFRS-RM scale are 0.85 and 0.84, respectively. The almost identical Cronbach alpha for any particular component demonstrates that adding the nocturnal hypoventilation item has no impact on the internal consistency of the rest of the scale.
Internal Consistency of Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised Modified
Construct Validity
The cross-sectional correlations between the ALSFRS-RM, the ALSFRS-R, their subscores, and FVC% are illustrated in Table 5. The correlation between the ALSFRS-RM and the FVC% (0.64) is markedly similar to the correlation between ALSFRS-R and FVC% (0.63). Within the respiratory function subscore, dyspnea and orthopnea have slightly better correlations to FVC% at 0.42 and 0.47, respectively, compared to item 12a (0.35).
Correlation Between Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised Modified, Nocturnal Hypoventilation Score, Subscales, and FVC%
Decline in Functional Subscores Over Time
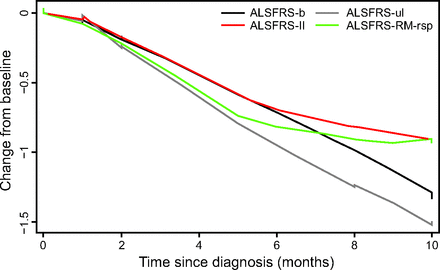
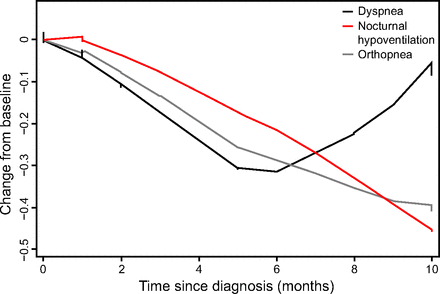
The decline in the individual subscores of the ALSFRS-RM within the first 10 months of diagnosis is displayed in Figure 1 Whereas the decline in ALSFRS-b and ALSFRS-ul over time is fairly linear, the rate of decline for the modified ALSFRS-RM-rsp, and to a lesser extent ALSFRS-ll, appears to display a floor effect 5–6 months after diagnosis. When dividing the ALSFRS-RM-rsp into its subcomponents, the orthopnea and nocturnal hypoventilation item scores demonstrate a steady decline, whereas the dyspnea score paradoxically improves after 6 months (Fig. 2).
Cross-sectional mean change from baseline to 10 months for each of the subscores of the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised Modified (ALSFRS-RM). b = bulbar, ul = upper limb, ll = lower limb, rsp = respiratory function.
Cross-sectional mean change from baseline to 10 months for the subcomponents of the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised Modified-respiratory function score.
Nocturnal Hypoventilation Item and Corresponding FVC%
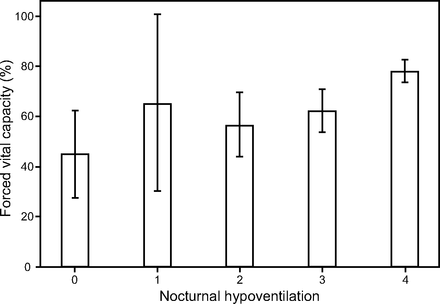
The disaggregated relationship between the FVC% and the individual values of item 12a is illustrated in Figure 3. There is a clear, positive relationship between FVC% and the nocturnal hypoventilation item score. The figure shows that individuals with all 4 symptoms assessed by item 12a (nocturnal hypoventilation item score = 0) have a mean FVC% of 44.9% (95% CI 30.4–59.4), whereas individuals with no symptoms (nocturnal hypoventilation item score = 4) have a mean FVC% of 78.2% (95% CI 73.7–82.7). The FVC% for a nocturnal hypoventilation item score of 4 is significantly higher than for the other scores (P < .001), but there is no statistically significant difference in FVC% between the other categories of item 12a. In addition, Figure 3 demonstrates that a nocturnal hypoventilation item score of ≤ 3 corresponds to an FVC% of 65% or less, with the upper 95% CI < 80%. The exception is a score of 1, where a wide CI is likely to be a result of few data points (Supplementary Table 1, see related supplemental material at http://rc.rcjournal.com).
Mean percentage (± 95% CI) of predicted FVC across nocturnal hypoventilation item values.
Discussion
The treatment of respiratory failure in ALS with NIV is standard practice, as it improves symptoms of nocturnal hypoventilation and quality of life.15 However, many individuals, especially in low- and middle-income countries, do not have access to this treatment modality. Because access to NIV is integral to the accurate use of item 12 within the ALSFRS-R, an alternative item is required when assessing individuals with respiratory failure without access to NIV. This study explored the utility of such an alternative item, whereas still retaining the option to assess individuals who are using NIV by means of the existing item 12. This is not a novel concept, as the current ALSFRS-R already provides a similar substitute question for upper-limb function in individuals with or without a gastrostomy.18
Our results suggest that the addition of an alternative item 12 to the existing ALSFRS-R may be a viable option for application in individuals not using ventilatory support. The new nocturnal hypoventilation item appears to reflect deterioration in respiratory function more accurately in individuals not using NIV. This is evident in Figure 2, which displays the ALSFRS-RM-rsp divided into its individual items over time. The nocturnal hypoventilation item score declines steadily as the disease progresses, whereas the original item 12 would remain constant at a score of 4 in individuals not using NIV.
The ALSFRS-RM maintains internal consistency when the alternative item 12 (item 12a) is employed, and Cronbach alpha is comparable to previous studies examining internal consistency of the ALSFRS and ALSFRS-R.3,4 Although a correlation of 0.35 between item 12a and FVC% appears low, it is approximately double that of the correlation between item 12 on the ALSFRS-R and FVC% (0.18), as found by Cederbaum et al.3 This should be interpreted with caution, as the Cederbaum study was conducted in a different population with a larger sample size (n = 387). However, these results would suggest that item 12a correlates at least as well with the FVC% as item 12 of the ALSFRS-R. In addition, the correlation of the ALSFRS-RM-rsp to the FVC% (0.42) was similar to that of the ALSFRS-R-rsp (0.44) (Table 5).
The results of this study may have an additional application in terms of the management of respiratory insufficiency. Although international consensus has not been reached on when to initiate ventilatory support, North American and European treatment guidelines suggest that NIV initiation should be considered when FVC% is < 50% or < 80% with additional symptoms of respiratory compromise.19 Our finding that a nocturnal hypoventilation item score of 3 is a marker of an FVC% of < 80% suggests that the presence of any one or more of these nocturnal hypoventilation symptoms imply an FVC% < 80%. This may have important implications for NIV initiation, particularly where access to formal respiratory function testing is limited. However, this application needs to be confirmed in an appropriately designed study.
A finding of this study that requires further investigation is the apparent floor effect of the ALSFRS-RM-rsp 5–6 months after diagnosis, as illustrated in Figure 1. The explanation for this phenomenon is not immediately apparent, although a floor effect can be anticipated in advanced disease with low ALSFRS-R scores. However, the floor effect in this subscore was seen 5–6 months after diagnosis and at a change from baseline ALSFRS-RM-rsp of < 1 point. A possible explanation for this result is that there may not be a linear relationship between the degree of respiratory insufficiency and the number of nocturnal hypoventilation symptoms.
Of additional interest is the observation that the dyspnea score values start improving after 6 months. As suggested by others, this may be related to decreased mobility and metabolic demand generating fewer respiratory symptoms.8 However, this finding will require further investigation by means of an appropriately designed study, the results of which may be helpful to further refine the respiratory subscore of the ALSFRS-R.
Our study has several limitations. First and foremost is the relatively small sample size and number of data points, especially in the 0–2 nocturnal hypoventilation item score categories. This is particularly evident in the group with a score of 1, which may have led to the aberrant finding of a higher than expected FVC% with very wide 95% CIs. However, in view of the correlation between a nocturnal hypoventilation item score of < 4 and an FVC% < 80%, it should be kept in mind that many individuals in these categories would have been excluded from this study because of NIV use. A second important limitation is the fact that this is only an exploratory study, and we were not able to validate our findings in a prospective cohort study. Such a study would require a relatively large number of participants and should ideally be a multi-center collaborative effort. Another possible limitation of the study relates to the objective measurement of respiratory insufficiency. A wider spectrum and potentially more sensitive measurements of respiratory function, such as maximum inspiratory pressure, maximum expiratory pressure, and sniff nasal pressure, would have provided additional and potentially more accurate parameters against which nocturnal hypoventilation symptoms could be measured.2 However, these measurements are not freely accessible for routine care in a resource-constrained environment such as ours. Nocturnal carbon dioxide measurement (eg, transcutaneous or end tidal) would probably be ideal but falls outside the scope of an out-patient clinic–based study such as ours. Furthermore, FVC% is widely used as an objective measure of respiratory function in neuromuscular disorders2,3,15,19 and as such continues to be a valuable parameter for comparison between studies.
Contrary to our expectations, the modified item 12 did not lead to a better correlation between the respiratory subscore and FVC%. The reason for this is not evident from our data but is likely related to the overall relatively poor correlation between FVC and symptoms of respiratory failure. Indeed, as is evident from supplementary Figure 1 and Figure 2 (see related supplementary materials at http://www.rc.rcjournal.com), there are numerous data points where participants with very low FVC% values have a normal ALSFRS-R-rsp and ALSFRS-RM-rsp subscore. Previous studies have also documented similarly modest correlations between patient-reported outcome measures of respiratory function and both FVC and slow vital capacity.19,20 It appears evident that patient-reported outcome measures are not reliable indicators of true respiratory function, but they remain valuable tools to assess quality of life, can be assessed remotely, and represent a patient-centered approach.
Conclusions
We propose that the ALSFRS-RM should be considered as an alternative scale to assess respiratory function in individuals with ALS who do not use NIV. The alternative item 12 may identify individuals with respiratory insufficiency that may have been missed by the original item 12. Furthermore, a score of < 4 on the nocturnal hypoventilation item (item 12a) may be an indicator of an FVC% < 80% and could potentially assist in early identification of individuals who may require NIV.
Footnotes
- Franclo Henning MD PhD, Division of Neurology, Faculty of Medicine and Health Sciences, Stellenbosch University, PO Box 19063, Tygerberg, 7505, Cape Town, Western Cape, South Africa. E-mail: fhenning{at}sun.ac.za
Supplementary material related to this paper is available at http://rc.rcjournal.com.
The authors have disclosed no conflicts of interest.
The study was conducted at Division of Neurology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, Western Cape, South Africa.
- Copyright © 2022 by Daedalus Enterprises