Abstract
BACKGROUND: Open respiratory secretion suctioning with a catheter causes pain and tracheobronchial mucosal injury in intubated patients. The goal of mechanical insufflation-exsufflation (MI-E) is to move secretions proximally and noninvasively by generating a high peak expiratory air flow. Nebulized hypertonic saline with hyaluronic acid (HS-HA) may facilitate suctioning by hydration. We assessed the safety and tolerance of a single session of airway clearance with MI-E and HS-HA in critically ill intubated patients.
METHODS: Adults with a cuffed artificial airway were randomized to (1) open suctioning, (2) open suctioning after HS-HA, (3) MI-E, or (4) MI-E with HS-HA. Adverse events, pain and sedation/agitation scores, and respiratory and hemodynamic variables were collected before, during, and 5-min and 60-min post intervention.
RESULTS: One-hundred twenty subjects were enrolled and completed the study. Median (interquartile range [IQR]) Acute Physiology and Chronic Health Evaluation II (APACHE II) score was 22 (16–28); median (IQR) age was 69.0 (57.0–75.7) y, and 90 (75%) were male. Baseline respiratory and hemodynamic variables were comparable. Adverse events occurred in 30 subjects (25%), with no between-group differences. Behavioral pain equivalents and Richmond Agitation-Sedation Scale were higher during suctioning in groups 1 (P < .001) and 2 (P < .001). Independent predictive variables for higher pain and agitation/sedation scores were study groups 1 and 2 and simultaneous analgosedation, respectively. Noradrenaline infusion rates were lower at 60 min in groups 2 and 4. PaO2/FIO2 had decreased at 5 min after open suctioning in group 1 and increased at 60 min in group 3.
CONCLUSIONS: We observed no difference in adverse events. MI-E avoids pain and agitation.
Introduction
Respiratory secretion suctioning (RSS) is an essential component of respiratory care in patients with an artificial airway.1 Conventional catheter suctioning is associated with significant pain,2,3 as well as with other local complications1 attributed to irritation, trauma, and suctioning of the tracheal mucosa being impacted by the tip of the catheter.
Mechanical insufflation-exsufflation (MI-E) is a potential noninvasive alternative applied for decades as an integral part of respiratory care with an oronasal interface in non-intubated patients with neuromuscular degenerative diseases since its initial description in an animal model and in humans.4,5 MI-E moves secretions proximally by generating a rapid outward air flow, analogous to a spontaneous cough. In patients with an endotracheal tube6 or tracheostomy cannula,7 MI-E produces a higher volume of mucus than conventional catheter suctioning and has been recommended for airway clearance8 in intubated patients.
Nebulized hypertonic saline is used for hydration of airway surface liquid and fluidification of respiratory secretions to facilitate mucociliary clearance in cystic fibrosis and bronchiectasis.9 It has been associated with improved lung function and less frequent exacerbations.10 In vitro and experimental data show antimicrobial and anti-inflammatory lung-protective effects.11,12 Tolerability of hypertonic saline solution is improved by its combination with hyaluronic acid.13,-,16
Advantages of the individual or combined use of MI-E and hypertonic saline with hyaluronic acid (HS-HA) might include improved tolerability and efficacy of noninvasive airway clearance. The combination may improve efficacy of airway clearance by MI-E of better hydrated fluidified secretions. Studies with safety of both measures as a primary objective in intubated critically ill subjects are not available, except for an uncontrolled preliminary experience with MI-E.17
The primary goals of the present trial were the comparative occurrence of adverse events and impact on pain and agitation-sedation scales of a single session of the study interventions in a mixed, unselected group of critically ill subjects. Secondary objectives were respiratory and cardiovascular variables and time from inclusion to extubation or spontaneous breathing. Results of this study have partially been reported in abstract form.18
QUICK LOOK
Current knowledge
Catheter secretion suctioning in intubated patients in the ICU is a necessary procedure for airway clearance to avoid the complications of accumulation of mucus such as impaired gas exchange, atelectasis, and respiratory tract infection. The maneuver, however, causes pain and trauma to the tracheobronchial mucosa.
What this paper contributes to our knowledge
We evaluated the safety and tolerance of mechanical insufflation-exsufflation (MI-E) to aspirate lung secretions in 120 subjects in combination with a nebulized hypertonic saline-hyaluronic acid solution. We observed a significant prevention of pain and improved tolerance of MI-E compared to conventional catheter suctioning, and a similar incidence of adverse events.
Methods
Study Design
We performed a prospective, open-label, randomized controlled study with a factorial design in subjects with an artificial airway requiring respiratory secretion suctioning. A factorial design was chosen to test for interaction of MI-E and nebulized HS-HA (7% sodium chloride solution with 0.1% sodium hyaluronate [Hyaneb, Chiesi, Spain]).19 The 4 study groups were (1) catheter open suctioning, (2) catheter open suctioning after nebulization of HS-HA, (3) MI-E, and (4) MI-E with nebulization of HS-HA.
Setting and Research Approvals
The trial was conducted at the Critical Care Department of Hospital Clínico San Carlos, Madrid, Spain, after approval by the local ethics committee (code number: 18/253-R_X). We followed the Consolidated Standards of Reporting Trials (CONSORT)-Outcomes 2022 extension20 of the 2010 statement.21 Informed consent was obtained from the subjects or their legal authorized representatives prior to trial enrollment.
Study Population and Study Procedures
Inclusion criteria were ≥ 18 y old; a cuffed endotracheal tube or tracheostomy cannula; on any assisted mode of ventilation or spontaneously breathing and requiring suctioning, regardless of the degree of respiratory failure, primary or secondary acute lung injury, atelectasis, tracheobronchitis or pneumonia, intracranial hypertension, hemodynamic stability, or any additional organ dysfunctions. The need for suctioning was established by suggestive respiratory sounds or auscultation, ventilator display curves,22 or visible mucus. Exclusion criteria were hemoptysis, bronchospasm, barotrauma, suspected unmonitored intracranial hypertension; uncontrolled muscular contractions (eg, tremor or myoclonus), and pregnancy (Fig. 1). After obtaining informed consent, sequentially numbered closed envelopes containing a computer-generated randomization sequence of the 4 study groups were opened.
Catheter open secretion suctioning in groups 1 and 2 followed local protocol and Spanish National Ventilator-Associated Pneumonia Prevention Guidelines.23 We applied MI-E in groups 3 and 4 with an airway clearance device (CoughAssist E70, Philips Ibérica S.A.U., Madrid, Spain) with a bacterial heat-moisture exchange filter (Intersurgical Inter-Therm HMEF with luer port, Intersurgical, Wokingham, United Kingdom) connected to the device output and a single-limb corrugated tubing between the filter and the artificial airway (see related supplementary materials at http://www.rcjournal.com). MI-E inhale-exhale pressures were +50 and −50 cm H2O, respectively, which is more than the recommended ± 40 cm H2O to overcome artificial airway resistance to air flow24 and based on recently published clinical experience17,25 and our routine clinical settings. Suctioning was considered productive if secretions were visible in the catheter or the proximal segment of the endotracheal tube or tracheostomy cannula in groups 3 and 4. In the latter case, secretions were aspirated with a sterile catheter introduced only for shallow suctioning, that is, with the catheter tip remaining in the artificial airway.26
Subjects were temporarily disconnected from the ventilator for open suctioning and the duration of the study maneuver and reconnected and returned to their baseline ventilatory status, without any change in settings, mode, or FIO2.
Data Collection
Clinical characteristics at ICU admission were collected with significant prior respiratory conditions defined as meeting COPD, asthma, or heavy smoking criteria. Respiratory category was defined at inclusion as (1) weaning, (2) PaO2/FIO2 > 300 mm Hg, (3) PaO2/FIO2 200–300 mm Hg, (4) PaO2/FIO2 100–200 mm Hg, and (5) PaO2/FIO2 < 100 mm Hg. The following data were obtained before and 5 min and 60 min after the study intervention: ventilation status (spontaneous breathing, CPAP, or mechanically ventilated); ventilator parameters and calculated dynamic and static compliance; respiratory and hemodynamic variables and arterial or venous blood gas values; noradrenalin infusion rates; analgesic; sedative drugs; and, if available, intracranial pressure (ICP) and bispectral index readings. Daily routine laboratory parameters were reviewed over the following 48 h to check for adverse events.
Adverse events were assessed from the start of the study procedure and at 5 min and 60 min and defined as (adopted from27) bronchospasm (bronchodilator needed), pneumothorax and atelectasis (as per daily chest radiographs until ICU discharge), desaturation (pulse-oximetry decrease > 5%) and hemoptysis and hypotension (> 30% decrease in systolic blood pressure [SBP] or > 10% increase in noradrenaline infusion from baseline), hypertension (> 30% increase in SBP or > 10% decrease in noradrenaline infusion from baseline), tachycardia (> 90 beats/min or > 30% increase in heart rate), bradycardia (< 40 beats/min), and supraventricular arrhythmias (anti-arrhythmic therapy needed). Subjects were kept under close clinical surveillance to detect any other complication.
Pain and sedation-agitation scale scores were calculated immediately before, during, and at 5 min and 60 min. We used a validated behavioral indicators of pain scale (ESCID, in its Spanish abbreviation)28 and the Richmond Agitation-Sedation Scale (RASS).29 Both systems are used routinely in our department, and scores were discussed and registered in agreement with the attending nurse. Cases were followed for occurrence of adverse events until ICU discharge or death.
Statistical Analysis
Comparisons of continuous variables were performed with one-way analysis of variance (ANOVA) or the Kruskal-Wallis test as appropriate. Repeated-measures ANOVA and Friedman ANOVA were applied to test for differences of values across study time points, with post hoc pairwise comparisons using the Bonferroni CI adjustment and the Greenhouse-Geisser correction for lack of sphericity.
Categorical variables were compared using the chi-square or Fisher tests. Two-way ANOVA was used to study the interaction of MI-E and HS-HA, as well as other potential significant factors for the main objectives of the study.
Stepwise multiple linear regression analysis was performed to find significant independent factors for the main outcome variables and to adjust for baseline variables. All statistical analyses were performed with IBM SPSS version 25.0 (IBM, Armonk, New York).
Outcome data for a precise sample size estimation are not available because, to the best of our knowledge, no detailed data on the incidence of MI-E–associated adverse events in critically ill intubated subjects have been published to date. We estimated that 120 subjects needed to be recruited to detect an absolute increase in AEs of 20%, from an incidence of 20% associated with catheter suctioning in 60 cases, to 40% in 60 cases under MI-E, with a margin of error of 10% and CI of 90%.
Results
Baseline Subject Characteristics
A total of 139 eligible candidates were evaluated for inclusion, 19 of whom met exclusion criteria, and 120 were randomized and completed the study (Fig. 1).
Median APACHE II score was 22 (16–28); 90 (75%) were male, and median age was 69.0 (57.0–75.7) y (Table 1). Characteristics at admission were well balanced between groups, with > 80% cases with an endotracheal tube, except for a higher incidence of respiratory insufficiency on admission in group 1. However, no differences in oxygenation and ventilatory support parameters were observed a median of 4.00 (2.00– 8.75) d later at study inclusion (Table 1).
Flow chart. HS-HA = 5 mL of 7% hypertonic saline with hyaluronic acid; MI-E = mechanical insufflation-exsufflation.
Demographic and Baseline Characteristics at ICU Admission and at Study Inclusion
Variables collected at enrollment were balanced between study groups, except for median body temperature (see related supplementary materials at http://www.rcjournal.com).
Mean MI-E insufflation tidal volumes in groups 3 and 4 were, respectively, 1,825.4 ± 668.5 mL and 1,944.2 ± 700.9 mL (mean absolute difference 118.0 [95% CI −480.0 to 241.5] mL, P = .43), and peak expiratory flows were 133.1 ± 19.8 L/min in group 3 and 131.3 ± 16.5 L/min in group 4 (mean absolute difference 1.8 [95% CI −13.8 to 17.4] L/min, P = .92).
Main Outcomes
Adverse events.
Total respiratory or cardiovascular adverse events occurred during suctioning in 30 subjects (25%) (Table 2), without differences between groups (P = .39). Rescue medication was given in 2, 1, 0, and 1 subjects in study groups 1–4, respectively (P = .56). All adverse events had resolved at the 60-min time point. Follow-up chest radiographs did not show complications. Days from study inclusion to extubation or spontaneous breathing, discharge or death, and total stay in the ICU were comparable (Table 2).
Adverse Events During Respiratory Secretion Suctioning
Pain and agitation-sedation assessment.
Median ESCID score rose significantly during suctioning in the whole study population (P < .001) and within each study group and decreased significantly at 5 min and 60 min (Fig. 2A, Table 3). Significant differences between study groups were identified only during suctioning (Fig. 2A, Table 3). Pairwise comparisons determined higher ESCID values for groups 1 and 2 (P < .001 for comparisons between group 1 and groups 3 and 4 and between group 2 and groups 3 and 4) but not between baseline and 5-min and 60-min time points. RASS scores were not different at any of the 4 time points (Fig. 2B, Table 3) but rose significantly from baseline during the suctioning procedure. Within-group analyses showed significant increases in groups 1 and 2 but not in groups 3 and 4.
Pain and agitation-sedation evaluation. A: Mean ± 95% CI behavioral indicators of pain scale (ESCID) score at (1) baseline, (2) during, (3) 5 min, and (4) 60 min after the respective secretion suctioning procedure. B: Mean ± 95% CI Richmond Agitation-Sedation Scale score. C: Mean ESCID scores ± 95% CI during the secretion suctioning procedure per study group in subjects with and without analgosedation. RSS = respiratory secretion suctioning, RASS = Richmond Agitation-Sedation Scale.
Between Study Group Comparisons of Behavioral Indicators of Pain Scale and Richmond Agitation-Sedation Scale Scores at Baseline, During, 5 Min, and 60 Min After the Study Intervention
The concurrent infusion of analgosedation in 95 (79.2%) subjects was associated with a lower ESCID score during RSS (P = .01) and showed interaction with the respective study intervention (P = .006) (Fig. 2C). Comparisons of ESCID score within each study group for subjects with and without analgosedation were significant only in group 2 (P = .006) (Fig. 2C). ESCID score correlated with MI-E (−0.43 [95% CI −1.74 to −0.82], P < .001) and analgosedation (−0.25 [95% CI −1.47 to −0.33], P = .002) in a model including MI-E and/or HS-HA; baseline analgosedation; and baseline infusion rates of midazolam, propofol, fentanyl, and remifentanil as independent variables.
Higher ESCID scores during suctioning correlated with study groups (1–4) (−0.41, P < .001), analgosedation (−0.31, P < .001), and respiratory category (1–5) (−0.21, P = .01), with no significant correlation between independent variables. Median ESCID scores during suctioning did not differ between respiratory categories, and 2-way ANOVA for study group and respiratory category did not differ for respiratory category.
The factorial ANOVA test confirmed the correlation of MI-E and a lower ESCID score (P < .001) and the absence of correlation with nebulized HS-HA. There was no interaction between MI-E and HS-HA for ESCID score.
Bispectral index disclosed no differences in distribution nor mean index at any of the 4 time points (see related supplementary materials at http://www.rcjournal.com) between groups in 21 subjects being monitored at the time of study inclusion. Mean bispectral index differed across time points (P = .007) for the whole study cohort. We observed similar mean values at baseline (45.50 ± 3.16) and during the procedure (45.00 ± 3.16), a higher mean bispectral index at 5 min (56.86 ± 4.17, P = .007), and a partial decrease to the 60-min mean index (50.43 ± 17.66, not significant to baseline and during procedure).
ICP monitoring was present in 15 subjects. We did not observe differences between study groups at any time point. Mean ICP stayed within normal limits across time points. We observed a transient ICP of 32 mm Hg in one subject in group 2 during catheter suctioning, with subsequent ICP < 20 mm Hg at 5 min and 60 min.
Secondary Outcomes
Hemodynamic and respiratory parameters.
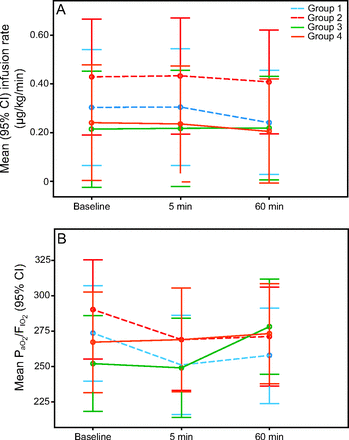
Mean heart rate, blood pressure, and noradrenaline infusion rates did not differ at baseline and 5-min and 60-min evaluations (see related supplementary materials at http://www.rcjournal.com). Across time points, pairwise comparisons disclosed reductions of noradrenaline infusion rates for the whole study population due to significant decreases in groups 2 and 4 (see related supplementary materials at http://www.rcjournal.com) from baseline to 60 min (mean difference −0.028 [95% CI −0.065 to 0.008], P < .001) and from 5 min to 60 min after the study intervention (mean difference −0.029 [95% CI −0.066 to 0.008], P < .001).
Hemodynamic and respiratory evaluation. A: Mean ± 95% CI noradrenalin infusion rates in µg/kg/min at baseline, 5 min, and 60 min after the respiratory secretion suctioning procedure. B: Mean ± 95% CI PaO2/FIO2.
There were no differences in ventilator modes at 60 min (P = .57) or PEEP or static and dynamic compliances at 5 min and 60 min, both between and within study groups nor in arterial and venous pH, PCO2, and PaO2/FIO2 at 5 min and 60 min for the whole study population. Mean PaO2/FIO2 decreased in group 1 at 5 min (P = .008), and an increase was seen at 60 min in group 3 (P = .01) (see related supplementary materials at http://www.rcjournal.com). No changes in this variable were found within groups 2 and 4.
Discussion
The results of our randomized controlled study suggest that a single session of RSS with MI-E and a nebulized solution of HS-HA is safe in intubated critically ill subjects. MI-E was associated with less pain and improved tolerance.
This study compares to the incidence and type of adverse events associated with MI-E and conventional catheter suctioning in a mixed intubated subjects population. Adverse events are not mentioned in most previous trials performed in critically ill subjects.30,31 One controlled trial enrolling 180 intubated subjects reported no desaturation or hypotension during MI-E at +40/−40 cm H2O or in the control group.6 In a recent retrospective safety analysis comparing prophylactic MI-E to intermittent positive-pressure breathing after extubation,32 lung mechanics were significantly improved; the development of atelectasis, pneumonia, or pleural effusion was similar; and the incidence of chest pain was higher, with no differences in length of hospital and ICU stay. An uncontrolled series of 157 unweanable subjects with Duchenne muscular dystrophy were successfully treated with MI-E pressures of +40/−40 cm H2O “or more,” but the authors did not report on the occurrence of adverse events.33 MI-E is widely used chronically in home management of neuromuscular diseases and spinal cord injury. In a 4-y study reporting in detail on adverse events in 181 subjects mostly on prolonged ventilatory support,34 one episode of barotrauma and one case of abdominal distention occurred not attributed to MI-E. One subject did not tolerate insufflation despite pressure adjustments. Barotrauma has not been reported in any controlled study of MI-E in neuromuscular diseases35,36 or in mechanical ventilation.6,30,37,38 Anecdotal reports in subjects receiving chronic positive-pressure ventilation have been published.39,-,41
We did not identify any safety issues with the administration of nebulized HS-HA, in line with previous studies,9,15 nor did we observe positive or negative interaction with MI-E. In addition, no impact of HS-HA on behavioral pain equivalent scores was detected. The potential synergy of its combined application with MI-E or the anti-inflammatory and antimicrobial effects remain to be evaluated in the clinical setting.
Demonstrating improved tolerance of secretion suctioning with alternative noninvasive methods is facilitated by the significant morbidity associated with the conventional procedure. Introducing a suctioning catheter into the lower airway in study groups 1 and 2 caused substantial pain, as confirmed by significantly higher ESCID scores. Consequently, the simultaneous infusion of analgosedation was associated with a lower impact on pain score during aspiration. Our findings confirm previous clinical research where MI-E was less painful and better tolerated than conventional suctioning,42 and its associated procedural pain score came close to the most painful procedures and was mitigated by opioids.43 Catheter endotracheal suctioning–associated pain is highly relevant not only because of pain intensity but also because it is one of the most frequently performed procedures.43
The impact of RSS on respiratory and hemodynamic parameters was similar between groups, except for changes in noradrenaline infusion rates and PaO2/FIO2 observed at some of the study time points. An anti-inflammatory effect of nebulized HS-HA in groups 2 and 412 allowing reductions in vasopressor infusion rates is extremely unlikely after a single nebulization. Similarly, the effect of the 4-s recruitment maneuver or improved airway clearance associated with MI-E6,44 in group 3 may have improved PaO2/FIO2, compared to catheter suctioning, but needs further evaluation during repeated suctioning sessions. Short-term improvement in lung mechanics after MI-E has been observed by some authors,25,32 but not by others,37 like in the present study.
The limitations of our study relate to its open-label design and single-center nature. Whereas most of the variables collected are objective, the subjective interpretation of widely used and recommended45 behavioral equivalents of pain scales is not completely devoid of observer bias, which is not prevented by randomization. Electrophysiological monitoring tools may improve behavioral pain assessment, although measurements correlate with the Behavioral Pain Scale.46 To limit bias, we scored ESCID28 after discussion with experienced attending nurses. The scale has been validated in a multi-center study where results were adjusted for internal consistency, inter-rater and intra-rater reliability, and assessed for its correlation with the Behavioral Pain Scale.47 Of note, endotracheal suctioning was used as one of the nociceptive stimuli for discriminant validation of ESCID, the Behavioral Pain Scale, and a study evaluating 6 different pain scales applied in intubated adults.48
The economic and environmental impact and the effect on nursing work load of the application of MI-E and HS-HA were not evaluated in our study. Neither are costly, and after the initial assembly of components, MI-E with HS-HA nebulization only requires changing connections from the ventilator to the airway clearance device. Future developments may introduce further simplifications through integration in mechanical ventilators.
Finally, our results only refer to a single maneuver applying a specific protocol. Safety of multiple daily sessions over consecutive days should not be inferred. Previous randomized trials applying MI-E over several days6,30,36,37 did not observe any adverse events, but the evaluation of safety of prolonged use of MI-E and HS-HA, as well as their interaction, in intubated critically ill subjects requires further evaluation and should remain a primary objective in future multi-center clinical research.
Conclusions
The present randomized controlled study did not observe differences in adverse events related to a single session of MI-E and nebulized HS-HA. Noninvasive respiratory secretion clearance with MI-E was less painful than conventional open catheter suctioning. Future studies should address safety and efficacy of noninvasive alternatives.
Acknowledgments
The authors would like to thank the nursing and respiratory physiotherapy staff for their help and assistance during this study. We also acknowledge Neus Cantariño MSc (Trialance SCCL, Spain) for providing medical writing assistance.
Footnotes
- Correspondence: Miguel Sánchez-García MD PhD, Critical Care Department, Hospital Clínico San Carlos, Prof Martín Lagos s/n, Madrid 28040, Spain. E-mail: miguelsanchez.hcsc{at}gmail.com
Dr Sánchez-García discloses a relationship with Chiesi Spain. The remaining authors have disclosed no conflicts of interest.
The study was presented at the European Society of Intensive Care Medicine 32nd Annual Conference, held September 28–October 2, 2019, in Berlin, Germany.
Chiesi Spain S.A.U. provided financial support for medical writing. Philips Respironics Spain supplied the airway clearance device (CoughAssist E70). Neither Chiesi Spain nor Philips Respironics Spain had any influence in the design, data analysis, or writing of the manuscript of the study.
Supplementary material related to this paper is available at http://www.rcjournal.com.
ClinicalTrials.gov registration number NCT03940118, December 30, 2018.
- Copyright © 2024 by Daedalus Enterprises