Abstract
BACKGROUND: When treating acute respiratory failure, both hypoxemia and hyperoxemia should be avoided. SpO2 should be monitored closely and O2 flows adjusted accordingly. Achieving this goal might be easier with automated O2 titration compared with manual titration of fixed-flow O2. We evaluated the feasibility of using an automated O2 titration device in subjects treated for acute hypoxemic respiratory failure in a tertiary care hospital.
METHODS: Health-care workers received education and training about oxygen therapy, and were familiarized with an automated O2 titration device (FreeO2,). A coordinator was available from 8:00 am to 5:00 pm during weekdays to provide technical assistance. The ability of the device to maintain SpO2 within the prescribed therapeutic window was recorded. Basic clinical information was recorded.
RESULTS: Subjects were enrolled from November 2020 to August 2022. We trained 508 health-care workers on the use of automated O2 titration, which was finally used on 872 occasions in 763 subjects, distributed on the respiratory, COVID-19, and thoracic surgery wards, and in the emergency department. Clinical information could be retrieved for 609 subjects (80%) who were on the system for a median (interquartile range) of 3 (2-6) d, which represented 2,567 subject-days of clinical experience with the device. In the 82 subjects (14%) for whom this information was available, the system maintained SpO2 within the prescribed targets 89% of the time. Ninety-six subjects experienced clinical deterioration as defined by the need to be transferred to the ICU and/or requirement of high flow nasal oxygen but none of these events were judged to be related to the O2 device.
CONCLUSIONS: Automated O2 titration could be successfully implemented in hospitalized subjects with hypoxemic respiratory failure from various causes. This experience should foster further improvement of the device and recommendations for an optimized utilization.
- respiratory failure
- oxygen supplementation
- automated oxygen titration
- hypoxemia
- hyperoxemia
- oxygen saturation
Introduction
Oxygen supplementation is ubiquitous in hospitalized patients with hypoxemic respiratory failure. Traditionally, the main concern of clinicians was to alleviate hypoxemia with little concern for hyperoxemia, except in the neonatal population1 and in COPD, where avoidance of hyperoxia to protect against respiratory acidosis was recommended.2 However, the appreciation that hyperoxemia may also be harmful in other conditions, such as sepsis and myocardial infarction, and after emergency surgery,3 has led to recommendations that 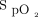 should be maintained within therapeutic zones that vary according to the cause of respiratory failure. For example, the British Thoracic Society has proposed
should be maintained within therapeutic zones that vary according to the cause of respiratory failure. For example, the British Thoracic Society has proposed 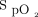 targets of 94 to 98% for patients who are the most acutely ill or 88 to 92% in those at risk of hypercapnic respiratory failure.4 Although guidelines for oxygen therapy are not uniform,4,-,8 their implications are that
targets of 94 to 98% for patients who are the most acutely ill or 88 to 92% in those at risk of hypercapnic respiratory failure.4 Although guidelines for oxygen therapy are not uniform,4,-,8 their implications are that 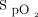 should be monitored closely and O2 flows adjusted frequently to maintain patients within a relatively narrow therapeutic window. Achieving this goal with manual O2 titration is labor intensive and often not feasible in the context of routine care.9 As such, it is common that patients are found outside the desired range of
should be monitored closely and O2 flows adjusted frequently to maintain patients within a relatively narrow therapeutic window. Achieving this goal with manual O2 titration is labor intensive and often not feasible in the context of routine care.9 As such, it is common that patients are found outside the desired range of 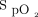 , both in the hypoxemic and hyperoxemic ranges.10-12
, both in the hypoxemic and hyperoxemic ranges.10-12
Automated O2 titration with devices that are based on closed-loop algorithms has been developed with the objective of maintaining 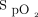 by providing continuous adjustment of O2 flow in the context of fluctuating oxygen requirements.13,14 These devices have been shown to increase the proportion of time spent in the desired
by providing continuous adjustment of O2 flow in the context of fluctuating oxygen requirements.13,14 These devices have been shown to increase the proportion of time spent in the desired 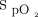 range in various clinical situations, including hospitalized subjects with COPD exacerbation,11,14 subjects in the emergency department,12 subjects after surgery,15,16 and subjects in COVID-19–related hypoxemic respiratory failure.17 Automated O2 titration has also been shown to be effective in situations in which rapid adjustments of O2 flows is required, for example, during exercise.18-20 It might also accelerate weaning from oxygen and hospital discharge,11,12,21 with potential reduction in hospitalization costs.22 Automated O2 titration has been implemented with high-flow nasal cannula, showing efficacy of the system to maintain
range in various clinical situations, including hospitalized subjects with COPD exacerbation,11,14 subjects in the emergency department,12 subjects after surgery,15,16 and subjects in COVID-19–related hypoxemic respiratory failure.17 Automated O2 titration has also been shown to be effective in situations in which rapid adjustments of O2 flows is required, for example, during exercise.18-20 It might also accelerate weaning from oxygen and hospital discharge,11,12,21 with potential reduction in hospitalization costs.22 Automated O2 titration has been implemented with high-flow nasal cannula, showing efficacy of the system to maintain 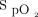 within the desired target zone during walking exercise in patients with COPD and in the context of hypoxemic respiratory failure.23,24 By reducing the requirement for direct contacts between hospital workers and patients because the O2 flows are automatically adjusted, the risk of transmission of contagious disease is reduced.25 This is an important consideration given the scarcity of hospital workers, overloaded health-care systems, and the high risk of hospital transmission of pathogens25,26 among health-care workers during the recent pandemic.27 The cumulative experience with automated O2 titration has been obtained in the research context, data also needs to be obtained in the clinical setting. We evaluated the feasibility of using this technology in the clinical care of subjects treated for acute hypoxemic respiratory failure in a tertiary care hospital.
within the desired target zone during walking exercise in patients with COPD and in the context of hypoxemic respiratory failure.23,24 By reducing the requirement for direct contacts between hospital workers and patients because the O2 flows are automatically adjusted, the risk of transmission of contagious disease is reduced.25 This is an important consideration given the scarcity of hospital workers, overloaded health-care systems, and the high risk of hospital transmission of pathogens25,26 among health-care workers during the recent pandemic.27 The cumulative experience with automated O2 titration has been obtained in the research context, data also needs to be obtained in the clinical setting. We evaluated the feasibility of using this technology in the clinical care of subjects treated for acute hypoxemic respiratory failure in a tertiary care hospital.
Quick look
Current Knowledge
It is recommended that 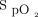 be closely monitored to avoid hypoxemia and hyperoxia when treating patients with acute respiratory failure. Maintaining
be closely monitored to avoid hypoxemia and hyperoxia when treating patients with acute respiratory failure. Maintaining 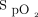 within a relatively narrow therapeutic window may be challenging with manual O2 titration, the standard of care in oxygen therapy. Automated O2 titration systems are currently being developed to reach this objective but their use is mostly limited to research settings.
within a relatively narrow therapeutic window may be challenging with manual O2 titration, the standard of care in oxygen therapy. Automated O2 titration systems are currently being developed to reach this objective but their use is mostly limited to research settings.
What This Paper Contributes to Our Knowledge
Automated O2 titration was implemented safely in the clinical care of subjects treated for hypoxemic respiratory failure in a tertiary care hospital. We also found that the ability of automated O2 titration to maintain 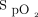 within the desired range in various diseases may also apply to “real life” clinical situations.
within the desired range in various diseases may also apply to “real life” clinical situations.
Methods
In 2019, a multidisciplinary committee advised hospital administrators on the possibility of implementing automated O2 titration within the context of clinical care. In 2020, 30 automated O2 titration devices were acquired by the hospital to treat patients in the emergency department or the hospital wards with various forms of hypoxemic respiratory failure, including COPD exacerbation, exacerbation of interstitial lung disease, viral (COVID-19) or bacterial pneumonia, and heart failure. This was done in the context of a prospective observational technological evaluation that took place between September 2020 and August 2022 at the Institut universitaire de cardiologie et de pneumologie de Québec, a 330-bed tertiary care, university-affiliated hospital with specialization in cardiology and respiratory medicine. When possible, clinical information, including etiology of respiratory failure, duration of O2 therapy, and hospital length of stay were noted. Prospectively collecting clinical data on the participants was exempted from ethics committee review by the institution because this was considered to be part of routine clinical care. The permission to use the clinical data anonymously for a scientific report was granted by the medical director of the hospital with a waiver of consent from the institutional ethics review board, when considering that this evaluation was done in the context of clinical care. The manufacturer had no role in data collection, interpretation, and presentation.
The automated O2 titration device used in this technological evaluation (FreeO2, Oxynov, Québec City, Canada) relies on continuous 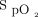 recording to feed a closed-loop algorithm, which allows automatic O2 flow titration to maintain
recording to feed a closed-loop algorithm, which allows automatic O2 flow titration to maintain 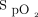 within a target window prescribed by the clinician.13 The device is coupled with a finger sensor linked to an embedded pulse oximeter (OEM III Module, Nonin Medical, Plymouth, Minnesota). The automated O2 titration device has 3 operating modes, the closed-loop mode being the primary operating mode, whereby O2 flow is changed based on measurement of
within a target window prescribed by the clinician.13 The device is coupled with a finger sensor linked to an embedded pulse oximeter (OEM III Module, Nonin Medical, Plymouth, Minnesota). The automated O2 titration device has 3 operating modes, the closed-loop mode being the primary operating mode, whereby O2 flow is changed based on measurement of 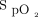 . In the closed-loop mode, O2 flow is automatically titrated based on the difference between the real-time
. In the closed-loop mode, O2 flow is automatically titrated based on the difference between the real-time 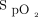 and the target value by using a proprietary proportional integral algorithm with an O2 flow command adjustment rate of once per second to achieve or maintain a pre-set
and the target value by using a proprietary proportional integral algorithm with an O2 flow command adjustment rate of once per second to achieve or maintain a pre-set 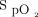 level. O2 flow is limited to 0.1 to 20.0 L/min. O2 flow may increase or decrease so as to maintain a stable
level. O2 flow is limited to 0.1 to 20.0 L/min. O2 flow may increase or decrease so as to maintain a stable 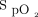 . The constant-flow mode is comparable with a standard O2 regulator, with the device only providing fixed O2 flow as set by the attending physician, between 0.1 and 20.0 L/min. In the acquisition mode, the device only monitors the oximeter readings (
. The constant-flow mode is comparable with a standard O2 regulator, with the device only providing fixed O2 flow as set by the attending physician, between 0.1 and 20.0 L/min. In the acquisition mode, the device only monitors the oximeter readings (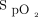 , breathing frequency, and heart rate) without any oxygen being provided. Data are visible on the front screen and captured in the device memory, from which it can be retrieved as long as the corresponding subject has been appropriately identified in the system (name, date of birth) when initiating therapy. However, this was not mandatory to initiate treatment with O2 titration device, and this step was often overlooked by health-care workers.
, breathing frequency, and heart rate) without any oxygen being provided. Data are visible on the front screen and captured in the device memory, from which it can be retrieved as long as the corresponding subject has been appropriately identified in the system (name, date of birth) when initiating therapy. However, this was not mandatory to initiate treatment with O2 titration device, and this step was often overlooked by health-care workers.
The implementation of automated O2 titration in clinical care of the hospital followed a multistep process to acclimate hospital workers to its use. A multidisciplinary implementation committee composed of nurses, respiratory therapists, physicians, a physiotherapist, and a patient representative oversaw the implementation of the devices and made recommendations about use (Table 1). A coordinator (PAB), helped by a senior nursing consultant (GPR) and one physician (FL), trained hospital workers (nurses, respiratory therapists, physicians) in various aspects of oxygen therapy and about the use of automated O2 titration. Training sessions were planned to be done in person but due to infection control measures in the COVID-19 pandemic, they were delivered remotely. The teaching material remained available for subsequent consultation if needed. The main topics covered were the following: (i) update on oxygen therapy, including the importance of avoiding hypoxemia and hyperoxemia; (ii) prescribing oxygen with lower and upper 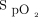 boundaries in a specific patient population and revision of the current guidelines; and (iii) practical use of automated O2 titration, including monitoring of subjects with the device, accurate patient selection, accurate
boundaries in a specific patient population and revision of the current guidelines; and (iii) practical use of automated O2 titration, including monitoring of subjects with the device, accurate patient selection, accurate 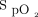 target, and potential issues associated with improper use of the device. A key learning objective was the detection of clinical deterioration with automated O2 titration. With fixed O2 flow, clinical deteriorations are detected when
target, and potential issues associated with improper use of the device. A key learning objective was the detection of clinical deterioration with automated O2 titration. With fixed O2 flow, clinical deteriorations are detected when 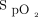 worsens. With automated O2 titration,
worsens. With automated O2 titration, 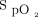 remains stable during disease instability. This is due to the intrinsic ability of automated O2 titration to maintain
remains stable during disease instability. This is due to the intrinsic ability of automated O2 titration to maintain 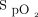 within the target zone as long as the maximum O2 flow allowed by the device is not surpassed. With automated O2 titration, clinical deteriorations, rather, are detected when requirements for O2 flow increase to maintain the target
within the target zone as long as the maximum O2 flow allowed by the device is not surpassed. With automated O2 titration, clinical deteriorations, rather, are detected when requirements for O2 flow increase to maintain the target 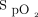 , which is a major change of practice for health-care workers.
, which is a major change of practice for health-care workers.
Composition and the Role of the Members of the Multidisciplinary Team
The coordinator (PAB) oversaw the use of automated O2 titration from 8:00 am to 5:00 pm during weekdays to address any concerns or technical questions with its use. In addition, 4 advanced practice nurses were available to accompany the hospital workers in the early weeks of the project when familiarity with the system had to be developed. A clinical protocol was developed by advanced practice nurses and respiratory therapists to address practical issues with the use of automated O2 titration (Table 2). This document covered the following topics: (i) contraindications to automated O2 titration, (ii) initiation of the device, (iii) setting of the clinical parameters of the device according to the clinical situation, (iv) how to mobilize subjects on the device, (v) clinical surveillance for nurses and respiratory therapists of patients on automated O2 titration, and (vi) weaning from automated O2 titration.
Clinical Care Protocol for the Installation of Automated O2 Titration
Patients admitted to the emergency department, respiratory and thoracic surgery wards, or COVID-19 unit with a diagnosis of hypoxemic respiratory failure were potentially eligible to be treated with automated O2 titration when a device was available. Patients with one or more of the following characteristics were not considered for automated O2 titration: (i) requirement for >8 L/min of O2 to obtain a 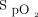 of ≥92% on the respiratory ward or emergency department or > 6 L/min of O2 to obtain an
of ≥92% on the respiratory ward or emergency department or > 6 L/min of O2 to obtain an 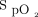 of ≥90% on the COVID-19 unit; (ii) requirement for noninvasive ventilation, high-flow nasal cannula, imminent endotracheal intubation, or cardiac arrest; (iii) impossibility of measuring
of ≥90% on the COVID-19 unit; (ii) requirement for noninvasive ventilation, high-flow nasal cannula, imminent endotracheal intubation, or cardiac arrest; (iii) impossibility of measuring 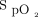 (poor peripheral perfusion, Raynaud, scleroderma); or (iv) agitation and/or absence of collaboration. We did not collect information about patients who were not considered for automated O2 titration.
(poor peripheral perfusion, Raynaud, scleroderma); or (iv) agitation and/or absence of collaboration. We did not collect information about patients who were not considered for automated O2 titration.
The proposed 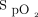 targets were 88 to 90% for COPD and 90 to 92% for other causes of hypoxemia but the final decision belonged to the physician. These
targets were 88 to 90% for COPD and 90 to 92% for other causes of hypoxemia but the final decision belonged to the physician. These 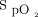 targets are lower than typically recommended4-8; they were selected based on our findings that the built-in oximeter that was used with the automated O2 titration device (OEM III Module; Nonin Medical) systematically underestimates arterial oxygen saturation (
targets are lower than typically recommended4-8; they were selected based on our findings that the built-in oximeter that was used with the automated O2 titration device (OEM III Module; Nonin Medical) systematically underestimates arterial oxygen saturation (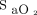 )28 and with the objective of avoiding hypoxemia and hyperoxemia. Weaning from automated O2 titration was proposed when O2 flow was ≤ 1 L/min. O2 flow,
)28 and with the objective of avoiding hypoxemia and hyperoxemia. Weaning from automated O2 titration was proposed when O2 flow was ≤ 1 L/min. O2 flow, 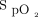 , and breathing frequency alarms were set at 8 L/min, 85%, and 40 breaths/min, respectively. The use of nasal cannula was recommended when O2 flows were < 5 L/min; oxygen masks (OxyMask [Medline, Northfield, Illinois] or a simple O2 mask) were used when O2 flows were ≥ 5 L/min or if it was more comfortable. Health-care workers had the opportunity to answer a short anonymous survey about their experience with automated O2 titration. The survey was available for one day, covering the 3 working shifts. The following questions were asked: (i) would you consider using an automated O2 titration device for your patients on oxygen, rarely, occasionally, or often; (ii) did you receive sufficient technical support for the use of automated O2 titration, yes or no; and (iii) on a 0 to 10 scale, 0 being completely useless and 10 being the most useful, how do you rate the clinical utility of automated O2 titration?
, and breathing frequency alarms were set at 8 L/min, 85%, and 40 breaths/min, respectively. The use of nasal cannula was recommended when O2 flows were < 5 L/min; oxygen masks (OxyMask [Medline, Northfield, Illinois] or a simple O2 mask) were used when O2 flows were ≥ 5 L/min or if it was more comfortable. Health-care workers had the opportunity to answer a short anonymous survey about their experience with automated O2 titration. The survey was available for one day, covering the 3 working shifts. The following questions were asked: (i) would you consider using an automated O2 titration device for your patients on oxygen, rarely, occasionally, or often; (ii) did you receive sufficient technical support for the use of automated O2 titration, yes or no; and (iii) on a 0 to 10 scale, 0 being completely useless and 10 being the most useful, how do you rate the clinical utility of automated O2 titration?
We report data to support the feasibility of using an automated O2 titration device in clinical practice, including the number of subjects for each hospital site (respiratory ward, COVID-19 unit, emergency department, thoracic surgery), etiology of respiratory failure, duration on automated O2 titration, hospital length of stay, 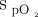 targets, and
targets, and 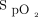 data. Clinical deterioration, defined as the need for high-flow nasal oxygen and/or transfer to the ICU, was recorded. Whether this was associated with the use of automated O2 titration or to progression of the underlying disease was documented from the medical chart or by discussing with the attending physician. We did not pre-specify the number of participants; this was determined by the duration of the project and by the availability of O2 titration devices. Some subjects were treated twice during the same hospitalization; when this happened, we only reported the first use. Categorical variables are presented as absolute or relative frequencies and were analyzed by using the Fisher exact test. Continuous variables are expressed as mean ± SD or as median (interquartile range [IQR]) according to the variable distribution. The between-group comparison for continuous variables was to perform a one-way analysis of variance. The normality assumption was verified with the Shapiro-Wilk tests by using residuals from the statistical model. The Brown and Forsythe variation of the Levene test statistic was used to verify the homogeneity of variances. Length of hospital stay was log-transformed to fulfill the normality and variance assumptions. We used the Wilcoxon rank-sum test to compare groups when the normality and variance assumptions were rejected. Statistical significance was present with a 2-tailed P < .05. Analyses were performed by using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
data. Clinical deterioration, defined as the need for high-flow nasal oxygen and/or transfer to the ICU, was recorded. Whether this was associated with the use of automated O2 titration or to progression of the underlying disease was documented from the medical chart or by discussing with the attending physician. We did not pre-specify the number of participants; this was determined by the duration of the project and by the availability of O2 titration devices. Some subjects were treated twice during the same hospitalization; when this happened, we only reported the first use. Categorical variables are presented as absolute or relative frequencies and were analyzed by using the Fisher exact test. Continuous variables are expressed as mean ± SD or as median (interquartile range [IQR]) according to the variable distribution. The between-group comparison for continuous variables was to perform a one-way analysis of variance. The normality assumption was verified with the Shapiro-Wilk tests by using residuals from the statistical model. The Brown and Forsythe variation of the Levene test statistic was used to verify the homogeneity of variances. Length of hospital stay was log-transformed to fulfill the normality and variance assumptions. We used the Wilcoxon rank-sum test to compare groups when the normality and variance assumptions were rejected. Statistical significance was present with a 2-tailed P < .05. Analyses were performed by using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
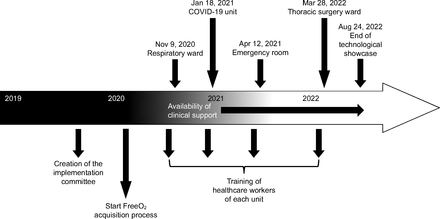
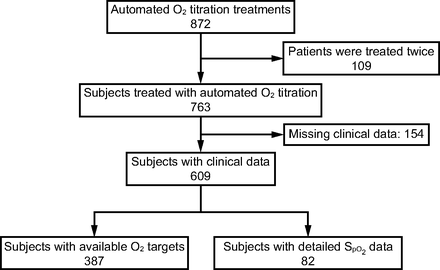
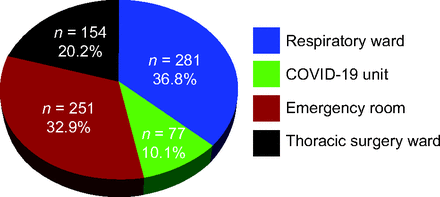
The automated O2 titration system began to be used on the respiratory ward in November 2020, on the COVID-19 unit in January 2021, in the emergency department on April 2021, and, finally, on the thoracic surgery ward on March 2022, with the last subject enrolled on August 24, 2022 (Fig. 1). A total of 508 health-care workers were trained to the use of automated O2 titration, including 399 nurses and respiratory therapists, 16 physiotherapists, 28 nurse assistants, 27 physicians (14 emergency physicians and 13 pulmonologists), and 38 medical residents. The flow of subjects is presented in Figure 2. O2 therapy was administered with the automated O2 titration device on 872 occasions in 763 subjects distributed on the respiratory, COVID-19, and thoracic surgery wards, and in the emergency department (Fig. 3). Of this number, we could retrieve clinical information on 609 subjects who were in the system for a median (IQR) of 3 (2-6) d, which represented 2,567 patient-days of clinical experience with the device. The characteristics of these subjects are presented in Table 3. The mean ± SD age of the subjects was 72 ± 12 y, median (IQR) hospital length of stay was 9 (6-15) d. Automated O2 titration was started at a median (IQR) of 0 (0-1) d after hospitalization, most subjects being previously treated with fixed-flow O2 at 2 L/min (IQR: 1–3 L/min).
Chronology of the implementation of the automated O2 titration device.
Flow chart.
Distribution of subjects on the automated O2 titration device.
Subject Characteristics
Three hundred two subjects of 609 subjects (49.6%) were weaned from oxygen while being on automated O2 titration, the remaining individuals had a few hours of fixed-flow O2 at low flows before O2 therapy was stopped. Due to disease worsening or instability, 42 subjects (7.0%) had to be transferred to the ICU and 81 subjects (13.3%) had to be treated with high-flow nasal cannula, including 54 who received this therapy outside the ICU. Thus, a total of 96 subjects (15.8%) reached the definition of clinical worsening. Under those circumstances, automated O2 titration was stopped according to the clinical care protocol. Attending physicians attributed these events to deterioration of the underlying condition and not to the use of the automated O2 titration device. With earlier versions of the software, the system was occasionally unstable and would stop automatically adjusting O2 flows for unclear reasons. When this occurred, the system automatically reverted to the constant-flow mode, delivering a O2 flow based on the analysis of the last 15 min of treatment. This functionality, which is also activated when the 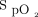 signal is interrupted or of poor quality, was created for safety purposes. These situations were immediately reported to the manufacturer and the software updated version 1.2.6, which was found to be reliable and working as expected.
signal is interrupted or of poor quality, was created for safety purposes. These situations were immediately reported to the manufacturer and the software updated version 1.2.6, which was found to be reliable and working as expected.
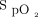 targets prescribed by clinicians were generally in agreement with those proposed by the implementation committee (Table 4). Based on the analysis of 387 subjects for whom the information could be retrieved from the device memory, the
targets prescribed by clinicians were generally in agreement with those proposed by the implementation committee (Table 4). Based on the analysis of 387 subjects for whom the information could be retrieved from the device memory, the 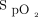 target was 88% to 90% in 306 subjects (79%), whereas
target was 88% to 90% in 306 subjects (79%), whereas 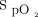 target ≥ 93% was used in 8 subjects (2.1%). The ability of automated O2 titration to maintain
target ≥ 93% was used in 8 subjects (2.1%). The ability of automated O2 titration to maintain 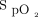 within the desired range could be assessed in 82 subjects for whom detailed
within the desired range could be assessed in 82 subjects for whom detailed 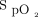 data could be retrieved from the device memory, 38 from the respiratory ward, 9 from the COVID-19 unit, and 35 from the emergency department. Baseline characteristics of these 82 subjects were similar to those of the 527 subjects for whom the oxygenation data were not available (Table 3). As can be seen in Table 5, the subjects were maintained within the
data could be retrieved from the device memory, 38 from the respiratory ward, 9 from the COVID-19 unit, and 35 from the emergency department. Baseline characteristics of these 82 subjects were similar to those of the 527 subjects for whom the oxygenation data were not available (Table 3). As can be seen in Table 5, the subjects were maintained within the 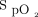 target zone for 89% of the time. Hypoxemia (
target zone for 89% of the time. Hypoxemia (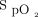 < 85%) occurred in < 5% of recording time, whereas hyperoxemia (
< 85%) occurred in < 5% of recording time, whereas hyperoxemia (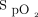 ≥ 98%) was found < 1% of recording time. Thirty-six of 508 health-care workers (7.1%) who used the automated O2 titration device filled out the survey about its utilization. Twenty-two of 36 health-care workers (61%) would often consider using automated O2 titration for patients on O2 therapy, 10 of 36 (28%) would do so occasionally, whereas 4 of 36 (11%) reported that they would rarely use it. Twenty-eight of 36 respondents (78%) considered that the technical support was sufficient and that they felt comfortable with the use of the device. The respondents gave the following device utility scores on a scale of 0 to 10: 5 (n = 2), 6 (n = 1), 7 (n = 5), 8 (n = 10), 9 (n = 5), and 10 (n = 13), with a mean ± SD utility rating score for automated O2 titration of 8.5 ± 1.5.
≥ 98%) was found < 1% of recording time. Thirty-six of 508 health-care workers (7.1%) who used the automated O2 titration device filled out the survey about its utilization. Twenty-two of 36 health-care workers (61%) would often consider using automated O2 titration for patients on O2 therapy, 10 of 36 (28%) would do so occasionally, whereas 4 of 36 (11%) reported that they would rarely use it. Twenty-eight of 36 respondents (78%) considered that the technical support was sufficient and that they felt comfortable with the use of the device. The respondents gave the following device utility scores on a scale of 0 to 10: 5 (n = 2), 6 (n = 1), 7 (n = 5), 8 (n = 10), 9 (n = 5), and 10 (n = 13), with a mean ± SD utility rating score for automated O2 titration of 8.5 ± 1.5.
Prescribed 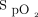 Targets in 387 Subjects on the Respiratory Ward, COVID-19 Unit, and Emergency Department
Targets in 387 Subjects on the Respiratory Ward, COVID-19 Unit, and Emergency Department
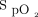 Data in 82 Admissions on the Respiratory Ward, COVID-19 Unit, and Emergency Department
Data in 82 Admissions on the Respiratory Ward, COVID-19 Unit, and Emergency Department
Discussion
We report our clinical experience with the feasibility of using an automated O2 titration device in subjects with acute respiratory failure requiring O2 therapy as a part of their routine clinical care. The cumulative data during this evaluation extends previous clinical trials in showing that the ability of automated O2 titration to maintain 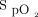 within the desired range in various diseases10-12,14-17 may also apply to “real-life” clinical situations. Clinical worsening, defined as the requirement for high-flow nasal oxygen and/or transfer to the ICU, in 96 subjects (15.8%) was attributed to progression of the underlying disease. Although not a unanimous choice among 36 health-care workers who responded to a short survey, the automated O2 titration device was felt to be useful and a positive experience was reported by the majority of users. This evaluation of the use of automated O2 titration in routine clinical care provided several learning opportunities that helped to address some frequently overlooked practical issues related to (i) oxygenation measurements with pulse oximetry and definition of optimal oxygenation with corresponding
within the desired range in various diseases10-12,14-17 may also apply to “real-life” clinical situations. Clinical worsening, defined as the requirement for high-flow nasal oxygen and/or transfer to the ICU, in 96 subjects (15.8%) was attributed to progression of the underlying disease. Although not a unanimous choice among 36 health-care workers who responded to a short survey, the automated O2 titration device was felt to be useful and a positive experience was reported by the majority of users. This evaluation of the use of automated O2 titration in routine clinical care provided several learning opportunities that helped to address some frequently overlooked practical issues related to (i) oxygenation measurements with pulse oximetry and definition of optimal oxygenation with corresponding 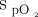 targets; (ii) organizational factors, including health-care workers training and supervision with the use of automated O2 titration; (iii) optimal utilization of automated O2 titration systems, and (iv) limitations in the accuracy of
targets; (ii) organizational factors, including health-care workers training and supervision with the use of automated O2 titration; (iii) optimal utilization of automated O2 titration systems, and (iv) limitations in the accuracy of 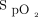 readings with currently available oximeters.
readings with currently available oximeters.
This technological evaluation provided an opportunity for nurses, physicians, respiratory therapists, and researchers from our institution to reflect and discuss various aspects of O2 therapy. The use of an automated O2 titration device, which forces clinicians to consider 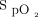 targets adapted to the clinical situation, may be helpful in implementing the recommendations that
targets adapted to the clinical situation, may be helpful in implementing the recommendations that 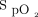 should be maintained in pre-specified therapeutic windows, avoiding both hypoxemia and hyperoxemia. Despite clear recommendations about the benefits of prescribing oxygen according to target ranges, clinical implementation of this approach is challenging and required numerous trainings, discussions, and feedback from clinicians. For example, in a recent audit of O2 therapy conducted in New Zealand,
should be maintained in pre-specified therapeutic windows, avoiding both hypoxemia and hyperoxemia. Despite clear recommendations about the benefits of prescribing oxygen according to target ranges, clinical implementation of this approach is challenging and required numerous trainings, discussions, and feedback from clinicians. For example, in a recent audit of O2 therapy conducted in New Zealand, 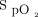 targets could be found in only 60% of hospitalized patients.29
targets could be found in only 60% of hospitalized patients.29
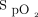 monitoring with the automated O2 titration device made us even more aware of the fluctuations in O2 needs in patients with acute respiratory failure, especially in patients with COVID-19, something that is not typically addressed by only intermittent (and often infrequent)
monitoring with the automated O2 titration device made us even more aware of the fluctuations in O2 needs in patients with acute respiratory failure, especially in patients with COVID-19, something that is not typically addressed by only intermittent (and often infrequent) 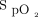 measurements. Automated O2 titration in response to
measurements. Automated O2 titration in response to 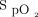 fluctuations was more effective than manual O2 titration to maintain subjects in the desirable
fluctuations was more effective than manual O2 titration to maintain subjects in the desirable 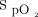 range as previously reported in a variety of clinical situations, such as in trauma subjects who are critically injured,10 in subjects admitted to the emergency department,12 after thoracic or abdominal surgery,15 those with COPD exacerbation,11,14 during exercise in subjects with chronic lung diseases,19 and in the pediatric population.30 Typically,
range as previously reported in a variety of clinical situations, such as in trauma subjects who are critically injured,10 in subjects admitted to the emergency department,12 after thoracic or abdominal surgery,15 those with COPD exacerbation,11,14 during exercise in subjects with chronic lung diseases,19 and in the pediatric population.30 Typically, 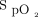 is maintained in the therapeutic zone 80% of the time with automated O2 titration compared with 40 to 55% of the time with manual O2 titration.10-12,14-17 When considering the importance of avoiding both hyperoxemia and hypoxemia,31 the goal of maintaining subjects within a safe
is maintained in the therapeutic zone 80% of the time with automated O2 titration compared with 40 to 55% of the time with manual O2 titration.10-12,14-17 When considering the importance of avoiding both hyperoxemia and hypoxemia,31 the goal of maintaining subjects within a safe 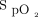 target zone is an argument in favor of automated O2 adjustment when caring for subjects with hypoxemic respiratory failure.
target zone is an argument in favor of automated O2 adjustment when caring for subjects with hypoxemic respiratory failure.
Our goal was to test the clinical implementation of an automated O2 titration device and not to make recommendations about O2 targets in specific conditions, and our experience should be interpreted in this context. The suggested 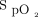 targets from the implementation committee (88 to 90% in the subjects with COPD, 90 to 92% in other situations) were lower than typically recommended, particularly in subjects other than those with COPD, with targets ranging between 90 and 98% have been recently proposed.4-8 The
targets from the implementation committee (88 to 90% in the subjects with COPD, 90 to 92% in other situations) were lower than typically recommended, particularly in subjects other than those with COPD, with targets ranging between 90 and 98% have been recently proposed.4-8 The 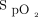 targets that were used took into consideration a study from our center that provided evidence that
targets that were used took into consideration a study from our center that provided evidence that 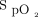 readings from the built-in Nonin oximeter, systematically underestimate
readings from the built-in Nonin oximeter, systematically underestimate 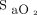 and are lower than those of other oximeters.28 In this study, it was found that
and are lower than those of other oximeters.28 In this study, it was found that 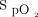 readings were, on average, 3% to 4% lower with the Nonin oximeter than with the Philips (Mississauga, Canada), Nellcor (Medtronic, Canada), or Masimo (Irvine, California) oximeters.28 One advantage of the Nonin oximeter is that an
readings were, on average, 3% to 4% lower with the Nonin oximeter than with the Philips (Mississauga, Canada), Nellcor (Medtronic, Canada), or Masimo (Irvine, California) oximeters.28 One advantage of the Nonin oximeter is that an 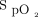 target of 90% allowed detection of all the hypoxemic episodes, whereas other tested pulse oximeters only detected 11 to 37% of these occurrences.28
target of 90% allowed detection of all the hypoxemic episodes, whereas other tested pulse oximeters only detected 11 to 37% of these occurrences.28
We, therefore, were confident that proposing 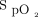 targets of 88% in COPD and 90% in other diseases with the system that was used in this technological evaluation would protect against hypoxemia and hyperoxemia while avoiding the risk of worsening hypercapnia in COPD and ensuring the safety of subjects.28 Although no blood gas data are available in the present report, a recent study from our group supports this practice by showing the an
targets of 88% in COPD and 90% in other diseases with the system that was used in this technological evaluation would protect against hypoxemia and hyperoxemia while avoiding the risk of worsening hypercapnia in COPD and ensuring the safety of subjects.28 Although no blood gas data are available in the present report, a recent study from our group supports this practice by showing the an 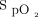 target of 90% with the Nonin oximeter is appropriate to protect against hypoxemia and hyperoxemia.32 Another consideration is that using higher targets could delay weaning of O2 and, therefore, unduly prolong the hospital stay, a situation that is more likely to occur when using an oximeter that systematically underestimates
target of 90% with the Nonin oximeter is appropriate to protect against hypoxemia and hyperoxemia.32 Another consideration is that using higher targets could delay weaning of O2 and, therefore, unduly prolong the hospital stay, a situation that is more likely to occur when using an oximeter that systematically underestimates 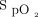 . Some clinicians showed some reluctance with our proposal for
. Some clinicians showed some reluctance with our proposal for 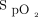 targets early in the technological evaluation, but, as clinical experience was gained and with appropriate teaching, most of them became comfortable with their use as shown in Table 4. The proposed
targets early in the technological evaluation, but, as clinical experience was gained and with appropriate teaching, most of them became comfortable with their use as shown in Table 4. The proposed 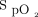 targets were applied primarily in people with light skin pigmentation who constitute the vast majority of the population treated in our hospital, an important consideration given the possibility to overestimate
targets were applied primarily in people with light skin pigmentation who constitute the vast majority of the population treated in our hospital, an important consideration given the possibility to overestimate 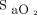 with pulse oximetry in people with dark skin pigmentation.33
with pulse oximetry in people with dark skin pigmentation.33
In theory, automated O2 titration, which allows for multiple adjustments of O2 flows, should be safer than relying on the current practice, in which it is challenging for health-care workers to precisely titrate O2 flows.9 However, we acknowledge that confirmation of this theory would require a formal randomized clinical trial. In patients with hypercapnic respiratory failure, the avoidance of hyperoxemia, which was infrequently observed (Table 5), should reduce the risk of worsening respiratory acidosis.
For automated O2 titration to be used safely, the premise that 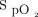 is an accurate surrogate of
is an accurate surrogate of 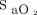 should be fulfilled and, unfortunately, this is not always the case.28,34,35 Indeed, oximetry readings should be viewed as approximating
should be fulfilled and, unfortunately, this is not always the case.28,34,35 Indeed, oximetry readings should be viewed as approximating 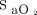 rather than considered as an accurate vital sign. Although imprecision of oximetry readings was reported years ago,34,35 this finding was largely unheeded by the medical community, with potential clinical consequences. For example, using an oximeter that systematically underestimates
rather than considered as an accurate vital sign. Although imprecision of oximetry readings was reported years ago,34,35 this finding was largely unheeded by the medical community, with potential clinical consequences. For example, using an oximeter that systematically underestimates 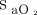 may lead to unduly high O2 flows if the
may lead to unduly high O2 flows if the 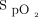 target is not adjusted accordingly.32 In people with dark skin pigmentation, overestimation of
target is not adjusted accordingly.32 In people with dark skin pigmentation, overestimation of 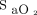 by pulse oximetry may lead to the occurrence of undetected hypoxemia and the risk of undertreatment based on certain
by pulse oximetry may lead to the occurrence of undetected hypoxemia and the risk of undertreatment based on certain 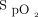 thresholds.33 Low perfusion and motion artifact may also compromise reliable
thresholds.33 Low perfusion and motion artifact may also compromise reliable 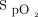 readings and thus any valid estimation of
readings and thus any valid estimation of 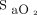 .35 These limitations are not specific to automated O2 titration devices because they apply to any situations in which O2 therapy is governed by
.35 These limitations are not specific to automated O2 titration devices because they apply to any situations in which O2 therapy is governed by 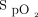 readings. What remains to be seen is to what extent imprecision of oximetry readings influence clinical outcomes; but, increased hospital mortality has been associated with the presence of undetected hypoxemia.36 While we are awaiting better oximeter accuracy, the understanding of current limitations of pulse oximetry should help to provide safer medical care.37
readings. What remains to be seen is to what extent imprecision of oximetry readings influence clinical outcomes; but, increased hospital mortality has been associated with the presence of undetected hypoxemia.36 While we are awaiting better oximeter accuracy, the understanding of current limitations of pulse oximetry should help to provide safer medical care.37
When this project was conceived in 2019, we did not foresee the use of an automated O2 titration device in subjects hospitalized with severe COVID-19 pneumonia. It turned out that such a system was useful in this circumstance because of the ability to continuously adjust O2 flows without direct contact between the subjects and the nurses and respiratory therapists. This offered added safety for the subjects and for hospital workers, likely reducing the risk of transmission of contagious disease.27 Another advantage of automated O2 adjustment was the reduction in the use of personal protective equipment, which was a major issue early in the pandemic due to scarcity. Our experience with automated oxygen titration is consistent with that of Danish investigators who reported a similarly positive experience in 20 subjects hospitalized with COVID-19 and with mild-to-moderate hypoxemic respiratory failure.17
Implementing new technology in clinical practice is challenging. Changing medical practice is a slow process, and there are multiple barriers to the adoption rate of health-care solutions.38,39 The importance of education and continued support and feedback for the medical team cannot be overemphasized. We also observed some clinical situations during which the behavior of the system was difficult to understand by the clinical team. On some occasions, subjects with COPD required relatively high O2 flows (4-6 L/min) to maintain target 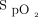 . These situations were uncomfortable for clinicians who are generally trained to avoid high O2 flows in COPD without appreciating that it is hyperoxemia and not high O2 flows per se that are responsible for worsening hypercapnia. In this context, the use of automated O2 titration may offer additional protection to subjects because as long as
. These situations were uncomfortable for clinicians who are generally trained to avoid high O2 flows in COPD without appreciating that it is hyperoxemia and not high O2 flows per se that are responsible for worsening hypercapnia. In this context, the use of automated O2 titration may offer additional protection to subjects because as long as 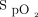 is maintained within a safe therapeutic window, hyperoxemia and worsening hypercapnia should not occur.
is maintained within a safe therapeutic window, hyperoxemia and worsening hypercapnia should not occur.
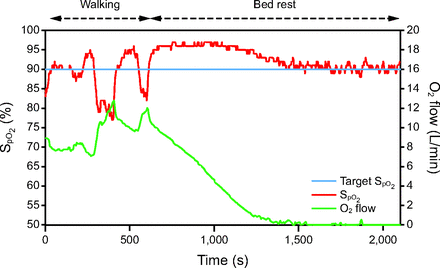
Some clinical situations were difficult to comprehend because of the time lag between oxygenation status (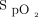 ) and incremental (or decremental) adjustments in O2 flows. One such situation is illustrated in Figure 4. A recovering subject resumed mild physical activities during which O2 desaturation was observed. This provoked a dip in
) and incremental (or decremental) adjustments in O2 flows. One such situation is illustrated in Figure 4. A recovering subject resumed mild physical activities during which O2 desaturation was observed. This provoked a dip in 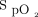 to which the automated O2 titration device responded by increasing O2 flow. On return to rest,
to which the automated O2 titration device responded by increasing O2 flow. On return to rest, 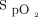 remained higher than the target value for some time while O2 flows progressively returned to lower values. For example, at 1,000 s, an O2 flow of 4 L/min could appear unexpected because the
remained higher than the target value for some time while O2 flows progressively returned to lower values. For example, at 1,000 s, an O2 flow of 4 L/min could appear unexpected because the 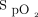 was above the target. In this situation, instantaneous reading of the physiologic parameters displayed on the automated O2 titration device, showing O2 flows that were higher than expected from the
was above the target. In this situation, instantaneous reading of the physiologic parameters displayed on the automated O2 titration device, showing O2 flows that were higher than expected from the 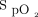 value could have mistakenly led to the conclusion that the device was not operating as intended, whereas this was simply a reflection of a time lag between the
value could have mistakenly led to the conclusion that the device was not operating as intended, whereas this was simply a reflection of a time lag between the 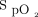 correction and the O2 flow response. Inspecting the
correction and the O2 flow response. Inspecting the 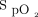 and O2 flow trend report from the device is crucial to understand the nature of the situation. Another example that requires some experience with the device is when the required O2 flow is low (approximately or < 1 L/min) and the patient is almost ready to be weaned from oxygen. Small fluctuations in
and O2 flow trend report from the device is crucial to understand the nature of the situation. Another example that requires some experience with the device is when the required O2 flow is low (approximately or < 1 L/min) and the patient is almost ready to be weaned from oxygen. Small fluctuations in 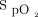 related to physical activities could induce a transient increase in O2 flow, which may prevent or slow O2 discontinuation. This situation may generally be resolved by simply stopping oxygen and monitoring the patient to ensure that
related to physical activities could induce a transient increase in O2 flow, which may prevent or slow O2 discontinuation. This situation may generally be resolved by simply stopping oxygen and monitoring the patient to ensure that 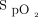 remains adequate.
remains adequate.
Relationship between 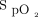 and O2 flow in one subject who was treated with the automated O2 titration device while transitioning from walking in the corridor to bed rest. Target
and O2 flow in one subject who was treated with the automated O2 titration device while transitioning from walking in the corridor to bed rest. Target 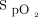 was set at 90%. During walking, a sudden fall in
was set at 90%. During walking, a sudden fall in 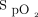 , down to 80% provoked a rapid rise in O2 flow aiming to return
, down to 80% provoked a rapid rise in O2 flow aiming to return 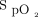 in the target zone. An overshoot in
in the target zone. An overshoot in 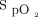 up to 95% was noted. Returning to bed resulted in reduced O2 needs as seen by a progressive decline in O2 flow down to 0 L/min over a 10-min period while
up to 95% was noted. Returning to bed resulted in reduced O2 needs as seen by a progressive decline in O2 flow down to 0 L/min over a 10-min period while 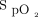 remained at the target value of 90%.
remained at the target value of 90%.
There are limitations to the present report that should be considered in interpreting the findings and their generalizability. The most obvious is that data were available only for a fraction of the subjects. There was a shortage of resources to collect detailed information in a systematic fashion as we are accustomed to in clinical research. For example, detailed 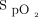 data could only be retrieved for 13% of subjects because many were not appropriately identified in the device, which made it impossible to match recorded oxygenation data with the corresponding subjects. Although the availability of detailed
data could only be retrieved for 13% of subjects because many were not appropriately identified in the device, which made it impossible to match recorded oxygenation data with the corresponding subjects. Although the availability of detailed 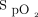 data in only a small portion of the subjects raises questions about the external validity of the findings, we were reassured that the proportion of time spent within the
data in only a small portion of the subjects raises questions about the external validity of the findings, we were reassured that the proportion of time spent within the 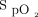 targets was similar to that previously observed in other clinical circumstances.10-12,14-17 Only a small number of nurses and respiratory therapists responded to the survey about the utilization of automated O2 titration. Therefore, its interpretation should be done cautiously. For example, it is possible that only those who had a positive experience with the device took the time to answer the survey. Lastly, the decision to use or not use the automated O2 titration system was made by the clinical team; we do not have data to estimate the proportion of hospitalized patients who require oxygen therapy treated with the device.
targets was similar to that previously observed in other clinical circumstances.10-12,14-17 Only a small number of nurses and respiratory therapists responded to the survey about the utilization of automated O2 titration. Therefore, its interpretation should be done cautiously. For example, it is possible that only those who had a positive experience with the device took the time to answer the survey. Lastly, the decision to use or not use the automated O2 titration system was made by the clinical team; we do not have data to estimate the proportion of hospitalized patients who require oxygen therapy treated with the device.
Conclusions
We found that automated O2 titration could be implemented safely in the context of routine clinical care in subjects with hypoxemic respiratory failure of various etiology. Based on this clinical experience and analysis of pilot data that suggest the possibility to reduce hospital length of stay with automated O2 titration,11 we encourage the conduct of randomized clinical trials that test the impact of automated O2 titration on hospital length of stay compared with the standard care in subjects with acute hypoxemia.
Acknowledgments
The authors thank the implementation team and health-care workers of our hospital for their contribution and who worked diligently to provide safe O2 therapy to the subjects. We also thank Serge Simard MSc, for his assistance with the statistical analyses.
Footnotes
- Correspondence: François Maltais MD, Centre de pneumologie, Institut universitaire de cardiologie et de pneumologie de Québec, 2725, chemin Ste-Foy, Québec, Canada G1V 4G5. E-mail: francois.maltais{at}med.ulaval.ca
Funding was provided by the Fondation de l’Institut universitaire de cardiologie et de pneumologie de Québec, Ville de Québec, Oxynov.
Drs Simon, Lacasse, Lellouche, and Maltais are shareholders of Oxynov, the maker of FreeO2; Dr Lellouche is co-founder of Oxynov.
See the Related Editorial on Page 1214
- Copyright © 2024 by Daedalus Enterprises