Abstract
BACKGROUND: Pediatric mechanical ventilation practice guidelines are not well established; therefore, the European Society for Paediatric and Neonatal Intensive Care (ESPNIC) developed consensus recommendations on pediatric mechanical ventilation management in 2017. However, the guideline’s applicability in different health care settings is unknown. This study aimed to determine the consensus on pediatric mechanical ventilation practices from Canadian respiratory therapists’ (RTs) perspectives and consensually validate aspects of the ESPNIC guideline.
METHODS: A 3-round modified electronic Delphi survey was conducted; contents were guided by ESPNIC. Participants were RTs with at least 5 years of experience working in standalone pediatric ICUs or units with dedicated pediatric intensive care beds across Canada. Round 1 collected open-text feedback, and subsequent rounds gathered feedback using a 6-point Likert scale. Consensus was defined as ≥ 75% agreement; if consensus was unmet, statements were revised for re-ranking in the subsequent round.
RESULTS: Fifty-two RTs from 14 different pediatric facilities participated in at least one of the 3 rounds. Rounds 1, 2, and 3 had a response rate of 80%, 93%, and 96%, respectively. A total of 59 practice statements achieved consensus by the end of round 3, categorized into 10 sections: (1) noninvasive ventilation and high-flow oxygen therapy, (2) tidal volume and inspiratory pressures, (3) breathing frequency and inspiratory times, (4) PEEP and FIO2, (5) advanced modes of ventilation, (6) weaning, (7) physiological targets, (8) monitoring, (9) general, and (10) equipment adjuncts. Cumulative text feedback guided the formation of the clinical remarks to supplement these practice statements.
CONCLUSIONS: This was the first study to survey RTs for their perspectives on the general practice of pediatric mechanical ventilation management in Canada, generally aligning with the ESPNIC guideline. These practice statements considered information from health organizations and institutes, supplemented with clinical remarks. Future studies are necessary to verify and understand these practices’ effectiveness.
Introduction
Pediatric mechanical ventilation is a fundamental therapy provided in the pediatric intensive care environment. However, mechanical ventilation research in critically ill children is often complicated by many factors, such as variation in size, maturity, and underlying conditions.1 Several guidelines and recommendations encourage standardized best practices, interprofessional collaboration, and decision making in pediatric mechanical ventilation management.2 As examples, the consensus guidelines from the Pediatric Acute Lung Injury Consensus Conferences3,4 and European Society of Paediatric and Neonatal Intensive Care mechanical ventilation guideline (the ESPNIC guideline1) are essential resources for practicing pediatric professionals. These guidelines3,4 were established by in-person consensus conferences and consisted of almost exclusively physician panel members.
The applicability of ESPNIC consensus statements1 into practice remains unknown especially in settings outside of Europe (recommendations were developed by a panel of 15 European physician experts). In Canada, respiratory therapists (RTs) have a large clinical role in mechanical ventilation management,5,6 yet the profession’s existence and decision-making involvement vary internationally. Canadian respiratory therapy programs train students in all aspects of respiratory care, including mechanical ventilation management, using a competency-based framework with national standards (https://www.csrt.com/rt-profession. Accessed August 24, 2021).5,6 Some studies have shown RT involvement in mechanical ventilation management can positively impact patient outcomes, particularly during the weaning process.7-10 The value of protocol-driven mechanical ventilation management is evident7,8,10-15; however, RTs remain infrequently listed as contributors to many published guidelines, even from regions where RTs have more widely adopted role. A systematic review by Ely et al2 published a directional guideline recommending the inclusion of non−physician health care providers in the development and implementation of mechanical ventilation protocols.
Empirical evidence guides health care practices and education, but not all questions can be answered by conventional research methods.16,17 Many treatment options may have insufficient evidence to support their use, but considerations for their use should not be discarded as clinicians require guidance on their safe application.18 Iterative consensus surveys are a valid way to collect information and perspectives to inform health care practices, especially on topics with insufficient or unknown evidence.16,19-21 One example is the Delphi survey technique, a common consensus method in health sciences that utilizes a well-informed expert panel to gather feedback to guide education and clinical practice.22-24 This feedback can be informed by multiple sources (including personal professional experiences) by using structured focus groups and/or surveys referred to as rounds.16,22 Cumulative expert knowledge and experiences provide validated opinions, rather than anecdotal claims,16 and are used to understand and clarify priorities, establish guidelines, identify gaps in knowledge, and to standardize practices and policies.17,18 Diverse information can be gathered from experts in a timely manner, especially when geographical constraints are present.22,24 Furthermore, the consensus product, such as a published guideline, should be continually reviewed and improved on through processes such as consensual validation.25 Experts’ practice deviations from published practice recommendations may highlight practical issues with application of guidelines to individualized care, knowledge translation of the recommendations, or the introduction of new practices and therapies that have yet to have evidence defining their use. The aims of consensual validation are likely to address these practical issues by determining the guideline’s content validity and identifying areas of improvements through additions, revisions, and deletions from the collective feedback of guideline authors and other targeted practitioners.25,26
The purpose of this study was to use a modified electronic Delphi (e-Delphi) method to determine the consensus on pediatric mechanical ventilation practices from the perspective of Canadian RTs and consensually validate components of the ESPNIC guideline recommendations.1,25,26
QUICK LOOK
Current Knowledge
There are limited pediatric mechanical ventilation practice guidelines to direct overall management in critically ill children. The European Society of Pediatric and Neonatal Intensive Care published a consensus pediatric mechanical ventilation management guideline in 2017; however, their international applicability across healthcare settings is unknown. Previous studies show the value of respiratory therapists' care experiences and role in protocol-driven mechanical ventilation management; however, they are rarely included in establishing respiratory care guidelines despite their large clinical role, especially in Canada and the USA.
What This Paper Contributes to Our Knowledge
Canadian respiratory therapists generally agree with the recommendations by the European Society of Pediatric and Neonatal Intensive Care. Their respiratory care experiences contributed to these practice statements that also incorporated elements from evidence-based national health and institutional care guidelines. Although the European guideline did not endorse the use of several modalities or therapies due to the lack of scientific data, our participants acknowledged this and emphasized that their use may be warranted in several clinical situations in collaboration with the interprofessional team.
Methods
Survey Design
The ESPNIC consensus statements1 informed the content of this survey, which followed a 3-round, modified e-Delphi method format.17,22 The Hospital for Sick Children (SickKids) and Ontario Tech University research ethics boards approved this study (number 1000064842 and number 15636, respectively).
Expert Participants and Recruitment
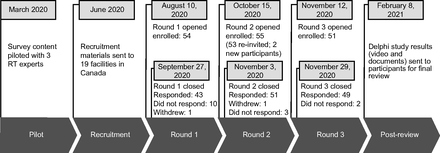
The expert panelists for this e-Delphi study were RTs with experience in pediatric mechanical ventilation management. Specifically, participants were eligible if they were a Canadian registered RT who (1) had at least 5 years of pediatric critical care experience, (2) had leadership experience either as staff involved in leadership activities or a formal leadership position, (3) provided written consent, and (4) were fluent in English (reading and writing). The goal was to recruit at least one RT from each pediatric hospital in Canada (15 standalone pediatric ICUs [PICUs], with another 7 hospitals with dedicated pediatric care beds27) for a minimum of 15 participants.17 Recruitment began in July 2020 and closed after round 2 ended in November 2020 (Fig. 1). Full details of the survey design and recruitment can be found in the supplementary files (see related supplementary materials at http://www.rcjournal.com).
Timeline of the 3-round Delphi survey from the pilot to post-Delphi review phase. Further details on the flow of participant engagement may be found in the supplementary materials. RT = respiratory therapist.
Survey Content and Development
The topics of this Delphi survey were derived from the ESPNIC recommendations,1 which consisted of 12 sections and 152 recommendations (some of these recommendations were repeated across several disease-specific sections). The purpose of this study was to summarize the general pediatric mechanical ventilation practices. Therefore, the 7 sections that outlined practice recommendations for different lung conditions (obstructive, restrictive, mixed)1 were reorganized, and recommendations that were repeated across different lung conditions were amalgamated. Sections on neuromuscular diseases and chronic and congenital lung conditions were omitted because focus of this study was on mechanical ventilation management in acute illness. Recommendations not explicitly related to mechanical ventilation (ie, chest physiotherapy) not commonly practiced in Canada and/or within the scope of RT practice were also omitted. Practices and topics that fall within the RT practices as outlined in the Canadian national competency (https://nartrb.ca/national-competency-profileframework. Accessed March 20, 2022) were incorporated into the survey. Some practice statements were revised to reflect a recent literature review and gray literature. Settings and target monitoring thresholds were refined to reflect national or international organizations’ recommendations, such as the Heart and Stroke Foundation of Canada’s Pediatric Advanced Life Support (PALS),28 Pediatric Acute Lung Injury Consensus Conference (PALICC),3 Extracorporeal Life Support Organization (ELSO; https://www.elso.org/Resources/Guidelines.aspx. Accessed March 3, 2020), Canadian Institute for Health Information (CIHI; https://www.cihi.ca/en. Accessed March 3, 2020), Canadian Patient Safety Institute (CPSI; https://www.patientsafetyinstitute.ca/en/toolsResources/psm/Pages/VAP-measurement.aspx. Accessed March 3, 2020), Children’s Hospitals’ Solution for Patient Safety (CHSPS; https://www.solutionsforpatientsafety.org. Accessed March 3, 2020), and National Institute of Health and National Heart, Lung, and Blood Institute ARDS Network clinical protocol (NIH-NHLBI ARDSNet; http://www.ardsnet.org/tools.shtml. Accessed March 3, 2020). A total of 64 recommendations were considered for review, reorganized, and revised for inclusion in our survey.
Three pediatric RTs (from SickKids, Toronto, Canada) piloted and provided feedback on a draft survey in collaboration with the research team. This process was to identify and refine statements to make them relevant to Canadian practices and reflect current practice guidelines from the aforementioned associations (PALICC, ELSO, CIHI, CPSI, CHSPS, ARDSNet). These revisions were incorporated into the final survey version for round 1, consisting of 53 statements. For round 1, participants were explicitly instructed to comment on the similarities or differences of their current practices and experiences to the survey items.
As part of round 1, participants also completed a demographics questionnaire that included personal characteristics (eg, age, sex, education), individual practice (eg, years of practice in pediatric critical care), and practice location.
Survey Analyses
Open feedback from round 1 was reviewed and guided the refinement of these statements for the next round. For subsequent rounds, participants rated their agreement using a 6-point Likert scale (with option 6-no comment if they did not have experience for the specific practice). The ranked responses were separated into 3 groups: Group 1 (disagree) included 1-strongly disagree and 2-disagree; group 2 (neutral) included 3-neutral; and group 3 (agree) included 4-agree and 5-strongly agree. Participants also were required to provide justification or rationale for their changes to statements when suggestions were made. These comments were reviewed by the authors (SQ, MN, KR), compared to existing literature, and statements were adjusted accordingly, if appropriate. Consensus was reached when 75% of the participants’ votes fell within one of the ranked groups.
Other Data Analyses
Descriptive statistics, including measures of central tendencies, response frequencies, dispersion, consensus percentage, and mean comparisons (including Wilcoxon rank-sum test across rounds 2 and 3), were performed using Microsoft Excel (Microsoft, Redmond, Washington) and IBM SPSS Statistics (IBM, Armonk, New York). P values < .05 were considered significant.
Results
Participant Demographics
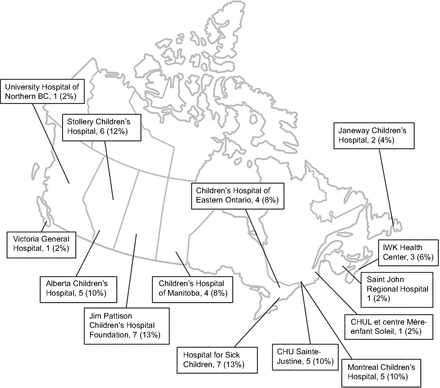
A total of 52 RTs completed at least one round of our survey (Fig. 1). The geographical representation of the participants included 12 facilities with standalone pediatric units and 2 with dedicated pediatric beds across Canada (Fig. 2). The mean (± SD) of pediatric RT experience was 15 (± 8.5) y. A full description of the demographics is available in Table 1.
Graphical representation of the Canadian expert panel. N = 52.
Participants’ Characteristics Across 14 Facilities
Delphi Rounds
An overview of the Delphi rounds and response rates is illustrated in Figure 1 In round 1, participants provided a large volume of written open-text feedback and suggested new statements on high-flow nasal cannula (HFNC) oxygen therapy, high-frequency jet ventilation (HFJV), including ventilator-associated pneumonia prevention practices and incorporating practices from guidelines by various health associations (eg, PALICC,3 PALS,28 ELSO). Thus, the feedback of round 1 resulted in the expansion of the survey to include 58 recommendations from 53 for round 2.
In round 2, 55 of 58 statements (95%) reached consensus: one statement between 75−80% (1.7%), 16 statements between 81−90% (28%), and 38 between 91−100% (66%). Three did not reach consensus (5%), and an additional 7 (10%) that reached consensuses but had considerable open-text feedback were revised (Supplementary Table 1, see related supplementary materials at http://www.rcjournal.com). One additional statement was added, resulting in a total of 11 statements for evaluation in round 3.
In round 3, all 11 statements received consensus (Supplementary Table 2, see related supplementary materials at http://www.rcjournal.com), with 9 increasing between 1−38% and one decreasing from 92% to 81% (the new statement achieved 100%). The full consensus practice statements included 59 items in 10 sections with clinical remarks (Table 2). In a post-Delphi review, participants did not provide any additional feedback.
Compilation of the Finalized Consensus Practice Statements
Pediatric Mechanical Ventilation Practice Consensus
Section 1: Noninvasive ventilation and high-flow oxygen therapy.
In agreement with ESPNIC, noninvasive ventilation (NIV) should be considered in combination with other medical therapies for children experiencing cardiopulmonary failure (Table 2). In addition, maximizing NIV patient-ventilator synchrony is crucial. Practice statements on HFNC oxygen therapy were not included in round 1 because ESPNIC did not provide recommendations on its use. However, participants identified HFNC oxygen therapy as a commonly used intervention in several clinical scenarios, and a practice statement was included for round 2. All NIV and HFNC practice statements achieved consensus by the end of round 2; however, the HFNC-specific statement received extensive feedback that warranted its revision and reassessment in round 3. The modified statement (number 1.6) was well accepted by participants and gained consensus by the end of round 3. For both NIV and HFNC oxygen therapy, ESPNIC and participants in this study strongly agreed that neither therapies should delay inevitable intubation.
Section 2: Tidal volume and inspiratory pressure.
Many participants agreed that the least amount of δ pressure (positive inspiratory pressure [PIP]-PEEP) with limited plateau pressures should be used to achieve target tidal volumes of 5−8 mL/kg, as recommended in the ESPNIC guideline. Participants mentioned tidal volumes may fall outside these target ranges to ensure adequate ventilation in specific clinical scenarios that should be discussed with the medical care team (number 2.2). Some participants emphasized that using ideal body weight (IBW) for target tidal volumes was ideal for certain weight or age, but these thresholds seem to vary across locations. This feedback was reflected in a clinical remark (number 2.1) to convey the importance of evaluating each child individually and discussing with the medical team to ensure target tidal volumes are not underestimated or overestimated.
Section 3: Breathing frequency and inspiratory time and Section 4: PEEP and FIO2.
Consensus was achieved for all items in these sections at the end of round 2. It was suggested that the children who require mechanical ventilation should have their settings routinely assessed and readjusted if required to optimize patient-ventilator synchrony, target tidal volumes, and minimize peak inspiratory pressures, and minute ventilation. Minimum PEEP levels slightly varied across practice locations, but ESPNIC and participants expected PEEP levels to be titrated to maintain adequate lung inflation and meet oxygenation goals. Similar to ESPNIC, our practice statement emphasized that in cases where pediatric ARDS was suspected PEEP titration should follow the guideline set by PALICC group.3
Section 5: Advanced modes of ventilation.
Many advanced modes of ventilation were not strongly recommended by ESPNIC due to limited evidence; however, both ESPNIC and the study participants (expressed through feedback during the rounds) indicated that different advanced modes (number 5.3 or 5.4) can be used in practice when appropriate. Thus, advanced modes were cautiously included in specific scenarios, and the practice statements were modified several times before reaching consensus in round 3. In the case of extracorporeal life support, participants endorsed the use of an established guideline by ELSO (96% consensus). Although all practice statements under section 5 eventually received consensus, a generic clinical remark for the whole advanced modes section is provided to emphasize their limited evidence.
Section 6: Weaning.
Specific weaning parameters were not provided by ESPNIC due to the lack of evidence. Therefore, practice statements in this section were generic in nature, suggesting clinicians consider spontaneous modes, routine weaning, and extubation readiness tests when patients are spontaneously breathing.
Section 7: Physiologic targets and Section 8: Monitoring.
Many of these practice statements were outlined in the ESPNIC guideline, and specific target thresholds were incorporated from other consensus recommendations such as PALICC3 and Heart and Stroke Foundation of Canada.28 It was important to emphasize that the physiologic targets and monitoring parameters are not definitive but could be tailored to fit the needs of the patients’ respiratory mechanics and pathologies. Participants strongly agreed with the incorporation of these elements, and clinical remarks were made to provide additional considerations toward physiologic targets and monitoring in different scenarios.
Section 9: General.
Many of the practice statements in this section were generic and could be applied to all clinical cases when children require mechanical ventilation, unless otherwise indicated. Though RTs are not commonly responsible for administering or managing sedation and muscle relaxants directly, it is within the Canadian scope of practice, and these practice statements were included to emphasize their role and impact on mechanical ventilation management. Though not mentioned in the ESPNIC guideline, participants emphasized the routine use of many organizational and working group guidelines and/or recommendations across many locations (number 9.5). The necessity of interprofessional collaboration and communication to facilitate mechanical ventilation management in children was emphasized; thus, this statement (number 9.7) was included for round 3, which achieved 100% consensus.
Section 10: Equipment adjuncts.
All practice statements in this section were from the ESPNIC guideline and were minimally revised to be more specific. Routine use of manual ventilation was not recommended by ESPNIC, but participants indicated that several scenarios would warrant its need (which were incorporated into the accompanying clinical remark). Indications for proximal flow sensors use appeared to be unclear and varied across practice locations. Though use of proximal flow sensors was recommended by ESPNIC, participants felt the statement was too vague. Thus, it was revised to suggest following the ventilator manufacturers’ guide or when tidal volumes were extremely small (< 10 mL). In addition, the need of proximal flow sensor use should be assessed, along with their measurement accuracy, and whether their measurements bring value to their overall mechanical ventilation management.
Discussion
This is the first study to use a modified e-Delphi survey to report the consensus of Canadian RTs’ pediatric mechanical ventilation practices and consensually validate aspects of the ESPNIC guideline. We achieved consensus for all practice statements by round 3. Broadly, participants agreed, indicating that they practiced similarly to the recommendations from the ESPNIC consensus guideline. Consensus was reached relatively quickly (55/58 statements [95%] in round 2), likely because the ESPNIC guideline was used as the foundation of the survey, with consideration to the literature and other evidence-based sources. Additional clinical remarks for these practice statements were from participants’ clinical experiences and feedback, likely reflective of current, common RT practices in Canadian PICUs. Whereas an interprofessional survey of practices would be of interest, we provided a cohesive perspective from RTs because they are one of the experts in pediatric mechanical ventilation management but are underrepresented participants in research.29
This study provided a form of consensual validation25 to the ESPNIC guideline. Other studies have utilized similar forms of validation. For example, a 2021 study engaged with nurses, physicians, and patients to consensually validate a renal replacement therapy care guideline derived from the literature.26 The care guideline was informed by the literature and congruent with patients’ treatment needs and clinicians’ priorities.26 Since our group of clinicians was not part of the development process of the ESPNIC guideline, this study shows that Canadian RT practices generally align or are consensually valid with European recommendations.
All the practice statements in our survey reached consensus by round 3; however, there were discrepancies across responses, highlighting the differences in pediatric mechanical ventilation practices across Canada. These practice statements required several refinements before consensus was achieved. The ESPNIC recommendations omitted statements on HFNC therapy due to the lack of evidence,1 but HFNC usage has increased over the years, and the timing of HFNC initiation relative to NIV continues to generate debate.30,31 Participants described HFNC as an option to alleviate work of breathing or treat respiratory failure in children with bronchiolitis, which is supported by existing publications.31-33 HFNC has been used in various clinical scenarios34 and practice locations (PICU and wards20) and has been shown to be safe and effective for treating bronchiolitis.33,35 An international survey identified HFNC as a frequently used modality with variable use across PICUs and a common preceding step to NIV.36 These surveys highlight the increasing popularity of HFNC and the necessity to evaluate its effectiveness in a broad range of clinical scenarios beyond bronchiolitis, as international guidelines on HFNC management do not exist.20,30,33,36,37 In a randomized controlled trial with 600 children, HFNC was compared to CPAP for postextubation respiratory support.38 HFNC did not meet the noninferiority criterion; HFNC was significantly associated with higher median time to respiratory support liberation and higher mortality.38 These findings support our HFNC practice statement that acknowledged HFNC is frequently used when NIV or intubation is not immediately indicated but also recognized HFNC is a distinct therapy from NIV and not interchangeable, nor should it delay escalation of care.30
There were also many comments regarding noninvasive and invasive neurally-adjusted ventilatory assist (NAVA) as an optional advanced mode of ventilation. ESPNIC did not recommend the routine use of NAVA, possibly due to the limited evidence and underutilization of NAVA in PICUs, its high costs,39,40 or lack of universal availability. In our study, about 25% of facilities indicated NAVA was unavailable, as NAVA is currently proprietary to Servo ventilators. We chose to incorporate participants’ feedback because highlighting NAVA use for future pediatric mechanical ventilation practice investigations is necessary because in recent years its use has increased along with evidence showing its effectiveness.40-44
In contrast to ESPNIC, our survey provided specific considerations regarding target tidal volumes, pressures, minute ventilation, and ventilator synchrony by referencing additional organizational guidelines (eg, PALICC,3 NIH-NHLBI ARDSNet). In our practice statements on tidal volumes, participants may have balanced the benefits, harms, or general applicability of various thresholds. Use of tidal volumes outside the physiologic range of 5−8 mL/kg IBW was uncommon; however, participants reported that tidal volumes outside this range may be used after collaborative discussions with the interprofessional team. Several studies have outlined that target tidal volumes < 5 mL/kg may be acceptable to achieve permissive hypercapnia and hypoxemia.3,4,45 Participants stated that tidal volumes are rarely set > 8 mL/kg but may be seen in children post-cardiac surgery, with lower breathing frequencies as a strategy to minimize mean airway pressure.45 We report the results of our Delphi study, though are cautious to suggest that high tidal volumes are best practices; but it may be seen in some children with cardiac pathologies but normal lung mechanics, resulting in low driving pressures with tidal volumes > 8 mL/kg. Overall, there is a lack of studies that have assessed and reported the effect of larger tidal volumes.46,47 We wanted to acknowledge participants’ remarks in regard to tidal volume individualization (> 8 mL/kg) as justified because it is supported by the literature,46,47 and we stress that target tidal volumes should be in the physiologic range, being mindful of universally lung-protective approaches.31
A similar statement (Table 2) (number 2.1) on tidal volume thresholds in patients with healthy lungs had decreased consensus from 92% to 81%, even with iterative changes. This may be because IBW was suggested when standardizing tidal volumes in all groups of children. Feedback revealed discrepancies regarding the use of IBW compared to actual weight and thresholds used to determine their tidal volumes. ESPNIC1 and PALICC3 guidelines recommend using IBW to normalize tidal volumes and minimize the risk of underventilating or overventilating children compared to using their actual weight.45,48 However, neither guideline provided direction as to how the IBW should be calculated, and PALICC recommended predicted body weight be used for all monitored values. Estimated target tidal volumes may need to be based on the measured or predicted body weight depending on the child’s body mass index.49 A few studies have shown lung volumes of overweight children are best estimated with predicted body weight, whereas normal-to-lower-weight children had lung volumes that aligned to their measured weight.47,49 To note, using IBW may estimate higher target tidal volumes compared to actual weight, except in overweight children, where tidal volumes set using IBW had decreased mortality.47 These inconsistencies on the use of standardized weights may be due to the lack of an universally accepted method and equation to calculate IBW in children, leading to differences in IBW values.50 In addition, there is still a debate on whether actual (instead of IBW) should be used and when.48,51 Furthermore, the differences in IBW and actual body weight appear to increase when children are > 25 kg.48 Based on our participants’ feedback, we summarized that IBW could be used for children > 10 kg and actual weight for those < 10 kg to minimize the overlap with neonatal thresholds. Guidance on which IBW calculation should be used remains unclear, and factors such as obesity and fluid overload should be considered when determining tidal volume.47,48,50
Two statements regarding advanced modes of ventilation received poor consensus in round 2 and generated variable feedback. Evidence surrounding the use of advanced modes of ventilation in children is relatively weak,52 and ESPNIC1 did not provide definitive recommendations on their routine use. Collective statements on different advanced ventilation modes were included in our practice statements as several participants wanted to acknowledge these options (eg, proportional assist ventilation [PAV], NAVA, automated weaning, airway pressure release ventilation [APRV]), despite their very limited use, reported harms, and lack of pediatric evidence.1,52 Originally, an HFJV practice statement was omitted for round 1, but participants mentioned that HFJV could be considered, though not regularly, in children (Table 2) (number 5.3). HFJV may be considered in children with acute respiratory failure or congenital heart diseases, as HFJV may improve ventilation and median pH.53,54 However, there are still uncertainties about HFJV for children as there are limited large and prospective studies describing its usefulness and effectiveness. APRV was another advanced mode that ESPNIC1 did not provide direction on. Recent studies have provided more understanding and knowledge on this mode; however, they appear to be conflicting.55-57 Studies have shown that APRV may have benefits such as decreased ventilation days,55,57 improved ventilation,55 oxygenation,56 and lung compliance.57 However, 2 prospective randomized studies in children with ARDS demonstrated that APRV was associated with increased mortality compared to low tidal volume ventilation57,58 and no significant benefits compared to high-frequency oscillatory,56 respectively. Of all the advanced modes of ventilation, there is minimal evidence to support the use of PAV in children. A systematic review and meta-analysis that evaluated the effects of PAV in adults and children reported no clinical trials in children.59 Overall, the medical team must discuss and balance the benefits, limitations, and available evidence when considering the use of any of these advanced modalities in children.
For equipment adjuncts, ESPNIC strongly recommended the use of proximal flow sensors,1 but participants mentioned they were not routinely used and may not be available in certain ventilators (in pediatric/adult patient categories). A previous study reported that tidal volumes measured by mechanical ventilators with compensation for tubing compliance or proximal flow sensors at the endotracheal tube were significantly lower than values measured using a calibrated pneumotachometer.60 This discrepancy may be a point of concern as underestimated volumes could have a clinical impact on the ventilation management, especially in children with ARDS.60 We acknowledged participants’ feedback on their use by stressing the use of proximal flow sensors should be based on local protocols, availability of devices, technical specifications of devices, availability of trained health care providers, and the interprofessional team’s decision (Table 2) (number 10.4). It is also important to note that many participants stated that proximal flow sensors were common in their local neonatal ICU (NICU) instead of PICU, indicating that care location may be a contextual factor in their use.
In round 2, it was universally emphasized that RTs are part of the interdisciplinary team and that mechanical ventilation management is collaborative. Thus, we added the practice statement (number 9.7). We chose to highlight this important statement, as it achieved 100% consensus in one round. We believe this highlights the interprofessional nature of mechanical ventilation management and the importance of collaboration with the interprofessional team.
In summary, these consensus statements are the first to describe pediatrics mechanical ventilation practices for critically ill children from the lens of Canadian RTs. Though the ESPNIC guideline did not provide strong recommendations for several therapies or management techniques, our survey suggests that their use exists as part of Canadian practices. Because there is limited evidence for some of these practices, clinical remarks were included to provide greater context and considerations in various scenarios. Furthermore, the increased use of therapies such as HFNC or NAVA demonstrates the need for future large multi-center studies to assess their effectiveness and safe use. It is important to note these practices were agreed upon by experienced PICU RTs and should only be performed by trained providers within their scope of practice and expertise and after discussion with the interprofessional team.
Strengths and Limitations
There are several strengths in this study. The survey contents were derived from the ESPNIC recommendations and incorporated elements supported by other health organizations or guidelines (eg, PALICC,3 PALS,28 ELSO, CIHI, NIH-NHLBI ARDSNet) pertinent to Canadian practices. Although we did not enroll participants from every pediatric center in Canada, our panel was demographically diverse with a total of 52 participants from 14 facilities across 9 provinces. Third, we had good survey response rates, increasing from 80% to 93% to 97% across rounds (typically, survey response rates decrease17,24). Lastly, we included a post-Delphi review stage of the finalized practice statements and to seek additional feedback from participants, as a method to enhance content validity and trustworthiness of the results.17,23 Both pilot and post-Delphi stages are not commonly performed or reported in previous Delphi studies.
There are also limitations to the study. We cannot exclude the possibility of response bias in our results; participants may have provided feedback that reflected local practice protocols; known best practices; or information read, heard, or witnessed instead of providing their own practice experience. Another potential source of bias may exist as 80% of our participants also practiced in the NICU, and responses could have reflected their neonatal practice or experiences. This study also sought feedback from participants during the Canadian first and second waves of the COVID-19 pandemic, and practice changes may have been implemented and influenced their perspectives in mechanical ventilation practices.
The participants in this study were limited to those who were fluent in English as we did not have the resources to translate our study materials into other languages. To mitigate this limitation, English-fluent participants were sought from health care facilities that used either English or French as their service language.
Conclusions
This is the first Delphi study utilizing RTs to create the first Canadian practice consensus on pediatric mechanical ventilation management for critically ill children. These broadly aligned with and were consensually validated with the ESPNIC guideline. All practice statements reached consensus by the end of round 3, and the final report included 59 items organized into 10 sections. In future investigations, considerations must be made for different institutional and jurisdictional practices, other health care practitioners, and emerging evidence.
Acknowledgments
This research would not have been possible without the feedback from the 3 SickKids RTs in our pilot phase and the RTs who volunteered to participate in this study.
Footnotes
- Correspondence: Mika L Nonoyama RRT PhD, Department of Respiratory Therapy, Hospital for Sick Children, 555 University Ave, Toronto, ON, M5G 1X8. E-mail: mika.nonoyama{at}ontariotechu.ca
Ms Quach discloses relationships with the Canadian Lung Association and the Lung Health Foundation. The authors remaining authors have disclosed no conflicts of interest.
Ms Quach presented a version of this research at the Canadian Society of Respiratory Therapists 2021 Annual Conference, held virtually May 5−7, 2021; and at the 31st Annual Meeting of the European Society of Paediatric and Neonatal Intensive Care, held virtually June 15−18, 2021.
Participant compensation supported by Dr Nonoyama’s research funding at The Hospital for Sick Children. Canadian Society of Respiratory Therapists provided collaboration and assistance in the recruitment phase.
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2022 by Daedalus Enterprises