Abstract
BACKGROUND: Intrapulmonary percussive ventilation (IPV) is used for airway clearance and delivery of aerosol medications, including bronchodilators. Despite the common use of IPV for drug delivery, few data are available regarding optimization of inhalation therapy with IPV. In this study, we investigated the influence of IPV setting parameters and lung mechanics on drug delivery via IPV alone.
METHODS: An IPV device was connected to a lung model via a trachea model and a flow analyzer. Albuterol nebulized from the IPV device was collected onto a filter attached between the trachea and lung models, and was quantitated by spectrophotometry (230 nm).
RESULTS: Albuterol delivery to the lung model was increased up to 2.1-fold, with decreasing percussion frequency. Decreasing percussion frequency concomitantly increased the tidal volume, and albuterol delivery was correlated with tidal volume (r = 0.91, P < .001). Airway resistance had a negative impact on albuterol delivery, whereas lung compliance had no significant effect. Increasing operational pressure increased albuterol delivery while increasing peak inspiratory pressure.
CONCLUSIONS: Albuterol delivery and tidal volume with IPV can be improved by maintaining low levels of percussion frequency and increasing operational pressure. When increasing operational pressure, the peak inspiratory pressure and airway resistance levels need to be carefully monitored for safe inhalation therapy with IPV.
Introduction
Intrapulmonary percussive ventilation (IPV) is high-frequency percussive ventilation that delivers small bursts of gas into the lungs for airway clearance.1 IPV can provide both ventilation and delivery of nebulized drugs simultaneously by its unique ventilation system.2 IPV is used in both children and adults for various diseases, including COPD,3–6 Duchenne muscular dystrophy,7 bronchiectasis,8 and cystic fibrosis.9,10 Therefore, IPV treatments need to be tailored for individual patients with differing respiratory conditions and clinical states. Although some intrapulmonary effects of setting parameters in IPV devices have been reported,11 it remains unknown how IPV parameters should be configured for optimal drug delivery in patients with different lung mechanics, including compliance (lung compliance [CL]) and airway resistance (Raw). Previously, drug delivery with IPV has only been investigated at a constant pressure and frequency12 or in a mechanical ventilator circuit.13 In clinical practice, however, drug delivery can be performed via IPV alone in patients without spontaneous breathing,14,15 and drug delivery optimization is not well understood.
In this study, we investigated the impact of alterations in the settings of the parameters of IPV (frequency and operational pressure) and lung mechanics (CL and Raw) on the efficiency of drug delivery and intrapulmonary effects. This in vitro study simulated the situation of patients on mechanical ventilation who received nebulized medication via IPV alone, with mechanical ventilation being withheld during IPV. Increased drug delivery and concomitant changes in the intrapulmonary effects with differing IPV parameter settings and lung mechanics provide insights into improved aerosol inhalation therapy with IPV in patients without spontaneous breathing.
QUICK LOOK
Current knowledge
Albuterol can be delivered via intrapulmonary percussive ventilation (IPV) in both patients who are spontaneously breathing and patients on mechanical ventilation. However, variations in the efficiency of drug delivery with differing IPV parameter settings and lung mechanics may impact patient response.
What this paper contributes to our knowledge
Albuterol delivery via IPV was increased with decreasing frequency and increasing operational pressure but was decreased at high airway resistance, irrespective of lung compliance. These IPV settings that improved albuterol delivery concomitantly increased tidal volume. Because peak inspiratory pressure markedly increased with increasing operational pressure at high airway resistance, careful monitoring is important to avoid lung injury.
Methods
Nebulization of Albuterol With IPV
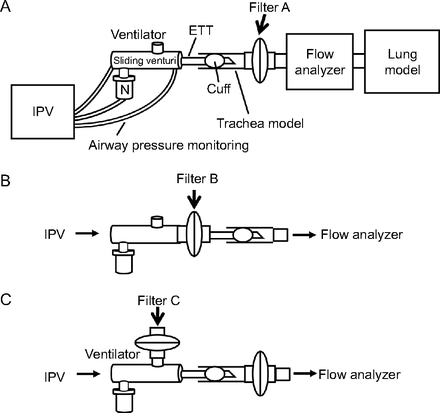
An IPV device (IPV-1C, A50007–1, A50010–3, Percussionaire, Sandpoint, Idaho) was connected to a trachea model (condenser tube, 20 mm × 30 cm, Percussionaire), FlowAnalyser (PF-300, IMT Medical, Buchs, Switzerland), and a lung model (Model 1600, Dual Adult TTL, Michigan Instruments, Grand Rapids, Michigan) (Fig. 1). A cuffed endotracheal tube (ETT) (8.0 mm × 36 cm) (TaperGuard, Medtronic, Dublin, Ireland) was placed to connect the sliding venturi (Percussionaire) to the trachea model with 25 cm H2O of cuff pressure (Fig. 1). One mL of albuterol solution (0.5% Ventolin, GlaxoSmithKline, London, United Kingdom) was diluted with 10 mL of saline solution and nebulized from the IPV device toward the lung model for 15 min.
Diagram of experimental setup for albuterol delivery with intrapulmonary percussive ventilation (IPV) and the positions of the filters for collection of albuterol. A: At the distal end of the trachea model. B: At the proximal end of the ETT. C: At the ventilator. ETT = endotracheal tube, N = nebulizer.
Configuration parameters of the IPV device were 20 or 40 psi of operational pressure and fully “easy” or “hard” mode of percussion. The easy and hard percussion settings correspond to frequencies of 300 and 100 cycles/min, respectively. The pulse/interval ratio setting was 1:2.5. The conditions of the lung model used were 20 or 100 mL/cm H2O of CL and 5 or 50 cm H2O/L/s of Raw. A filter in a cartridge housing (Inspiratory/Expiratory Breathing Circuit Filters, RT019, Fisher and Paykel, Auckland, New Zealand) to capture albuterol was placed between the trachea model and the FlowAnalyser, separated from the tip of the ETT by 2 cm (Filter A) (Fig. 1). The FlowAnalyser measured peak inspiratory flow, peak expiratory flow, PEEP, peak inspiratory pressure (PIP), and tidal volume (VT). These intrapulmonary effects were measured in triplicate 1 min after the start of IPV. To examine any deposition of albuterol outside the lung model, 2 additional filters were attached at the proximal end of the ETT (Filter B) and the ventilator of the sliding venturi (Filter C) (Fig. 1).
Measurement of Albuterol
All the albuterol solution was completely nebulized from the IPV device within 15 min of operation, and the filter cartridge was immediately removed from the circuit. The filter was immersed in 2 mL of 100% ethanol in a 15-mL Falcon tube (Corning, Corning, New York) and centrifuged at 200g for 10 min after elution with agitation for 2–3 h. The albuterol concentration (μg/mL) was determined at 230 nm with an ND-100 spectrophotometer (NanoDrop Technologies, Wilmington, Delaware)13,16 by using a standard curve of albuterol dissolved in ethanol. The minimum measurable concentration was 8 μg/mL. The efficiency of albuterol delivery was estimated as the following: percentage efficiency = (the amount of albuterol captured in a filter [μg]/5,000) × 100.
Rationale for Parameter Settings of the Lung Model
The Raw and CL values of healthy individuals are <5 cm H2O/L/s and >80 mL/cm H2O, respectively.17,18 In 24 subjects on ventilation and with pulmonary edema and chronic airway obstruction, Raw ranged from 8.0 ± 4.6 to 26.4 ± 13.4 cm H2O/L/s and CL was from 35 ± 5 to 66 ± 20 mL/cm H2O on day 1 from the onset of mechanical ventilation, and there was a subject who showed > 50 cm H2O/L/s of Raw due to obstructive lung disease.19 Another in vivo study showed that 13 subjects on ventilation and with acute lung injury had 7.2–17.2 cm H2O/L/s of Raw and 26.3–102 mL/cm H2O of CL.20 Hence, we mimicked these subjects on ventilation and with target symptoms for IPV therapy and configured the parameters of the lung model. The normal pulmonary conditions to simulate healthy lungs were 5 cm H2O/L/s of Raw and 100 mL/cm H2O of CL, whereas diseased pulmonary conditions 50 cm H2O/L/s of Raw and/or 20 mL/cm H2O of CL.
Statistical Analysis
Parameters were set in 16 combinations of frequency (easy vs hard mode) and operational pressure (20 vs 40 psi) in the IPV device, and CL (20 vs 100 cm H2O/mL) and Raw (5 vs 50 cm H2O/L/s) in the lung model. The amounts of albuterol deposited onto filters were measured in triplicate in these 16 combinations, and the 2-tailed Wilcoxon signed-rank test was performed to assess whether the amounts of albuterol delivered were changed between 2 different settings of each parameter. The correlation between VT and the amounts of albuterol deposited onto filters was evaluated by the Pearson product-moment correlation coefficient. All statistical analyses were conducted by using SPSS version 25.0 (IBM, Armonk, New York), and P values < .05 were considered statistically significant.
Results
Albuterol delivery and intrapulmonary effects in 16 combinations of frequency and operational pressure in the IPV device and Raw and CL in the lung model were determined as follows.
Albuterol Delivery Via IPV
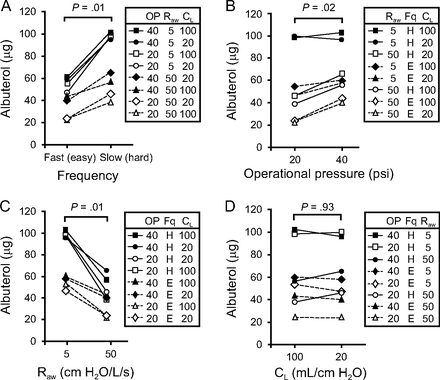
The amounts of albuterol in the eluates of the filters (Filter A) (Fig. 1) ranged from 23.7 to 102 μg (Fig. 2), which corresponded to 0.5–2.0% of the initial dose (5 mg) of albuterol nebulized from the IPV device. The parameters that achieved the maximum albuterol delivery were 40 psi of operational pressure and the hard mode of percussion under the normal pulmonary conditions of the lung model, that is, 5 cm H2O/L/s of Raw and 100 mL/cm H2O of CL. In contrast, the lowest albuterol delivery was observed at 20 psi, easy mode, and 50 cm H2O/L/s of Raw, irrespective of CL (Fig. 2D). Under the normal pulmonary conditions, 140 μg of albuterol (2.8% of the initial dose) was captured at proximal end of the ETT (Filter B) (Fig. 1) and 760.8 μg (15.2% of the initial dose) at the ventilator of the sliding venturi (Filter C) (Fig. 1). Therefore, these results indicated that the amounts of albuterol delivered to the ETT by the intake flow were ∼5 times lower than that removed from the ventilator by the counterflow.
Effect of intrapulmonary percussive ventilation (IPV) parameters and lung mechanics on albuterol delivery. The amounts of albuterol were compared between 2 different conditions of each parameter by using the 2-tailed Wilcoxon signed-rank test. A: Frequency (easy or hard). B: Operational pressure (20 or 40 psi). C: Airway resistance (Raw) (5 or 50 cm H2O/L/s). D: Lung compliance (CL) (20 or 100 mL/cm H2O).
Intrapulmonary Effects
VT.
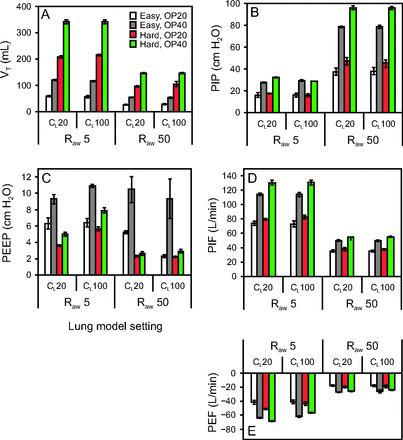
VT ranged from 27 to 341 mL. As previously reported,11 increasing operational pressure and decreasing frequencies increased VT (Fig. 3A). VT was also increased with decreasing Raw, irrespective of CL. VT was >200 mL at the hard mode and 5 cm H2O/L/s of Raw, even at 20 psi of operational pressure. The largest VT value was observed at the hard mode, 40 psi of operational pressure and 5 cm H2O/L/s of Raw, whereas the lowest VT value was at the easy mode, 20 psi of operational pressure and 50 cm H2O/L/s of Raw.
Intrapulmonary effects of intrapulmonary percussive ventilation (IPV) parameters and lung mechanics. A: Tidal volume (VT). B: Peak inspiratory pressure (PIP). C: PEEP. D: Peak inspiratory flow (PIF). E: Peak expiratory flow (PEF). OP = operational pressure (20 or 40 psi); Raw = airway resistance (5 or 50 cm H2O/L/s); CL = lung compliance (20 or 100 mL/cm H2O). The error bars represent SD of 3 independent experiments.
PIP.
PIP ranged from 15.6 to 95.8 cm H2O (Fig. 3B). PIP was increased with increasing operational pressure and Raw, irrespective of CL, and was shown to be >30 cm H2O at 50 cm H2O/L/s of Raw. The frequency had a marginal effect on PIP. The lowest PIP value was observed at 20 psi of operational pressure under normal pulmonary conditions. The highest PIP value was at the hard mode, 40 psi of operational pressure and 50 cm H2O/L/s of Raw.
PEEP.
PEEP ranged from 2.2 to 10.9 cm H2O (Fig. 3C). PEEP was increased with the easy mode and increased operational pressure. The highest PEEP value was at the easy mode and 40 psi of operational pressure under the normal pulmonary conditions. The lowest PEEP value was at the hard mode, 20 psi of operational pressure and 50 cm H2O/L/s of Raw and 100 mL/cm H2O of CL.
Peak Inspiratory Flow.
The highest positive value of pulse flow during inspiration (see supplementary Fig. 1 at http://www.rcjournal.com), peak inspiratory flow, ranged from 34.8 to 131.0 L/min (Fig. 3D). Peak inspiratory flow was increased with increasing operational pressure and decreasing Raw, irrespective of CL. The maximum peak inspiratory flow was observed at the hard mode and 40 psi of operational pressure under the normal pulmonary conditions. The minimum levels were at the easy mode, 20 psi of operational pressure and 50 cm H2O/L/s of Raw.
Peak Expiratory Flow.
The lowest negative value of pulse flow (supplementary Fig. 1 at http://www.rcjournal.com), peak expiratory flow, ranged from −68.3 to −17.6 L/min (Fig. 3E). The extent of the peak expiratory flow was increased with increasing operational pressure and decreasing Raw, as observed for peak inspiratory flow (Fig. 3D).
Discussion
Drug delivery via IPV is commonly performed in patients on mechanical ventilation, although the influence of IPV setting parameters on drug delivery has not been well understood. It also remains unclear how differing lung mechanics influence the efficacy of the IPV treatment. To our knowledge, this study was the first to show variations in the efficiency of drug delivery and intrapulmonary effects with differential IPV parameter settings and lung mechanics.
The maximum amount of albuterol (∼100 μg, 2% of the initial dose) was delivered at the hard mode of frequency (∼100 cycles/min) under the normal pulmonary conditions of the lung model, which was ∼2 times greater than that at the easy mode (∼300 cycles/min). With decreasing frequency from the easy to hard mode, the entrainment of gas from the nebulizer by the sliding venturi improved, which accounted for the increase in albuterol delivery (Fig. 2A) and VT (Fig. 3A; supplementary Fig. 2 at http://www.rcjournal.com). The relationship of VT with frequency was consistent with the Toussaint et al study.11 Increasing operational pressure also increased albuterol delivery (Fig. 2B). Interestingly, CL was found to have no significant impact on albuterol delivery (Fig. 2D). It was likely that the lack of influence of CL was attributed to minibursts of air delivered by IPV with high frequency. The IPV-treated airways can be held inflated by the high-frequency gas pulse.3,4 By contrast, increased Raw had a negative impact on albuterol delivery (Fig. 2C). The decrease in albuterol delivery at high Raw was related to a decrease in the quantity of gas entrained by the venturi as a result of the increased downstream resistance. The same is true for the decrease in VT with increasing Raw (see supplementary Fig. 3 at http://www.rcjournal.com).
The maximum albuterol delivery was observed when VT was >200 mL (Fig. 4). It should be noted that albuterol delivery correlated with VT during IPV (r = 0.91, P < .001) (Fig. 4). Previously, the impact of VT on drug delivery has been reported as both positive and neutral by using jet nebulizers, a pressurized metered-dose inhaler, and IPV.13,21–25 O'Riordan et al,25 when using 4 types of jet nebulizers, reported that increasing VT from 700 to 1,000 mL resulted in up to a 25% increase in drug delivery. Fink et al23 found a progressive increase in drug delivery from a pressurized metered-dose inhaler with increasing VT from 100 to 800 mL. In contrast, Berlinski and Willis13 reported that changing the VT setting in a pediatric ventilator model from 100 to 200 mL did not increase drug delivery via IPV or jet nebulizer. However, the relationship between drug delivery and VT differed, depending on the position of IPV in the ventilator circuit. When IPV was placed at the humidifier (distal from the lung model), drug delivery to the lung model increased by ∼50% with increasing VT, although the amount of albuterol delivered was markedly lower with IPV at the humidifier than that at the Y-piece (proximal to the lung model).13 Although it seems likely that both VT and the distance between the aerosol generator and the lung model influences drug delivery via IPV, neither VT nor the distance affects drug delivery with a jet nebulizer.13 Thus, there is a complex relationship between drug delivery and VT. In our study, concomitant increases of VT and albuterol delivery were observed via IPV alone, which indicated that the IPV settings of frequency and operational pressure that improved albuterol delivery were effective in ventilation as well.
Relationship between tidal volume (VT) and albuterol delivery.
Only a small fraction of nebulized albuterol was emitted from the IPV device (Fig. 1B), as previously described.26 The aerosolized drug from the sliding venturi may be further deposited within the ETT and the trachea model, possibly due to turbulent flow (Fig. 1A and B).27 Thus, it seemed likely that the majority of albuterol remained inside the IPV device and was expelled from the ventilator (Fig. 1C). However, the efficiency of albuterol delivery in the study by Berlinski and Willis13 was 2 times higher (4%) than that in our study when IPV was placed at the Y-piece. Because the mechanical ventilation in their study provided VT of 100 mL or 200 mL at 20 breaths/min, the inspiratory flow from it may contribute to the drug delivery.13 In addition, the sliding venturi was different in that the ventilator was closed in their study and open in our study (see supplementary Fig. 4 at http://www.rcjournal.com). The presence or absence of the ventilator cap on the Phasitron housing may also influence the efficiency of drug delivery because a substantial amount of albuterol was expelled from the ventilator (Fig. 1C). When considering these differences in the experimental setup, the efficiency in albuterol delivery could not be directly compared between these 2 studies.13 Thus, it would be of interest to study the efficiency of drug delivery by the combination of mechanical ventilation settings with IPV settings based on the current study.
The clinical efficacy of albuterol delivery via IPV as conducted in this in vitro study was previously described for airway clearance in subjects with atelectasis or asthma on mechanical ventilation.14,15 The drug delivery via IPV involves the removal of the ventilator circuit tubing from the ETT adapter and the attachment of the IPV device's tubing to the ETT adapter, then albuterol is administered by inhalation during IPV for 10–20 min. Recently, the IPV treatment has also been performed to improve the viability and availability of lungs of brain-dead organ donors for lung transplantation.28,29 A study by Deakins and Chatburn14 used IPV settings at frequencies that ranged from 180 to 220 cycles/min and operational pressure equal to 15–30 cm H2O of the peak pressure for albuterol delivery in pediatric subjects with atelectasis. Results of our study suggested decreasing frequency to ∼100 cycles/min and increasing operational pressure to improve drug delivery, irrespective of CL. Importantly, it was reported that pulmonary congestion occurred after 20 min in healthy lungs of a rat ventilated at 45 cm H2O of PIP.30 Therefore, it is necessary to carefully maintain the airway plateau pressure <30 cm H2O during IPV for prevention of lung injury in patients with elevated Raw.31,32 Further in vivo studies on optimization of IPV parameter settings are needed for safe and effective IPV therapy in various lung diseases.
Limitation
This study was an in vitro experiment and could not be extrapolated to humans without caution because trachea and lung models mimic human lungs imperfectly. Although IPV may be superimposed on a conventional ventilator,33 this study did not consider the use of such ventilators to address the influence of the setting parameters of IPV alone on drug delivery and exclude potential influence of mechanical ventilation–related factors on drug delivery.13,34,35
Conclusions
We investigated the variations in the efficiency of drug delivery and the intrapulmonary effects with differential IPV parameter settings and lung mechanics. We showed that decreasing percussion frequency can increase drug delivery and VT. Raw had a negative impact on drug delivery, whereas CL had no significant influence. Effective parameter settings for albuterol delivery were the hard mode (lower frequency) and 20 or 40 psi of operational pressure. Because high operational pressure may result in excessive PIP gain, depending on Raw, it is important to monitor PIP with extreme caution in patients with high Raw.
Footnotes
- Correspondence: Yusuke Mimura MD, Department of Clinical Research, National Hospital Organization Yamaguchi-Ube Medical Center, 685 Higashi-Kiwa, Ube, 755-0241 Japan.
The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
See the Related Editorial on Page 612
- Copyright © 2019 by Daedalus Enterprises