Abstract
Asthma is a common chronic disease that affects both adults and children, and that continues to have a high economic burden. Asthma management guidelines were first developed nearly 30 years ago to standardize care, maintain asthma control, improve quality of life, maintain normal lung function, prevent exacerbations, and prevent asthma mortality. The two most common asthma guidelines used today were developed by the National Asthma Education and Prevention Program (NAEPP) Expert Panel Working Group and the Global Initiative for Asthma Science Committee. Both guiding documents use scientific methodology to standardize their approach for formulating recommendations based on pertinent literature. Before the 2020 National Asthma Education and Prevention Program (Expert Panel Report 4), nothing had been released since the 2007 guidelines, whereas the Global Initiative for Asthma publishes updates annually. Although each of these asthma strategies is similar, there are some noted differences. Over the years, the focus of asthma treatment has shifted from acute to chronic management. Frontline respiratory therapists and other health-care providers should have a good understanding of these 2 guiding references and how they can impact acute and chronic asthma management. The primary focus of this narrative is to look at the similarities and differences of these 2 guiding documents as they pertain to the 6 key questions identified by the Expert Panel of the National Asthma Education and Prevention Program.
Introduction
Asthma is a chronic condition estimated to affect 262 million adults and children globally.1 The global mortality from asthma is relatively low (1%), but the burden of treating and managing asthma remains high.2 In the United States, asthma affects 25 million people and direct health-care costs for uncontrolled asthma are ∼$300 billion with a total economic burden of $963 billion.3 There are 2 primary asthma resources to provide ongoing and comprehensive asthma diagnosis and management, recommendations to reduce the economic and social burden and improve health outcomes of asthma: the National Asthma Education and Prevention Program guideline (NAEPP)4 and the Global Initiative for Asthma report (GINA).5 The NAEPP was initiated in 1989 through the United States National Heart, Lung, and Blood Institute, Institutes of Health, to raise awareness of asthma, recognize signs and symptoms of asthma, ensure effective asthma control, and enhance the quality of life for those diagnosed with asthma within the US.4 The first NAEPP was released in 19916 and updated on an as-needed basis (1997, 2002, 2007, 2020)7-10 by using key questions to determine what content should be changed.4 GINA5 was established in 1993 in collaboration with the World Health Organization and the National Heart, Lung, and Blood Institute, National Institutes of Health to determine strategies for global asthma care. The first GINA11 was released in 1995 and updated annually by using recent scientific literature to guide recommendations. Both of these resources are used today to guide clinical practice for asthma management in the United States.
Evolution of Asthma Management
Over the years, advancements in science have led to changes in how health-care providers treat and manage asthma. In the 1960s and 1970s, asthma treatment focused on relief and prevention of bronchospasm. Then, in the 1980s and 1990s, prevention of allergen-induced bronchospasms and airway inflammation were addressed for asthma control. Today, because asthma is recognized as a heterogeneous disease, the focus has shifted to individual treatment strategies for asthma prevention and management. Several of the biggest shifts in asthma management identified both in the NAEPP10 and GINA12 include intermittent inhaled steroid use in viral-induced wheezing or mild asthma, intermittent maintenance and relief therapy in mild persistent asthma, and single maintenance and relief therapy for mild-to-severe asthma. Both the NAEPP10 and GINA12 now recognize that poor medication is common and can lead to worsening asthma outcomes and intermittent asthma control.
Scientific Methodology
The scientific methodology uses an objective and standardized approach to formulating recommendations based on reviewing the pertinent literature. The methodology and approach of the NAEPP10 and GINA12 are different, yet similar. Both the NAEPP Expert Panel7 and the GINA12 Science Committee are recognized leaders in asthma research and clinical practice. Both groups use the Grading of Recommendations Assessment, Development, and Evaluation criteria to address relevance to population, intervention, comparison, and outcomes.10,12 The NAEPP Expert Panel consists of asthma content experts, primary care clinicians, and experts in dissemination and implementation of health-care policies.10 The NAEPP Committee performed a systematic review of the literature on 6 priority topics through October 2018 conducted by the Agency for Healthcare Research and Quality. These 6 topics were determined to be the most important based on needs assessment.10 Before the 2020 NAEPP (Expert Panel Report 4),7 nothing had been released since the 2007 NAEPP (Expert Panel Report 3).9 The GINA12 Science Committee, meets twice a year in conjunction with the American Thoracic Society and European Respiratory Society to do an extensive review of published asthma research from the previous 18 months, then publishes their recommendations annually.
Review of the Literature
Intermittent Inhaled Corticosteroids in Younger Children
Asthma is one of the most common chronic diseases in children, affecting ∼7% of the population.13 Recurrent wheeze also occurs in a large portion of younger children with a viral respiratory tract infection1; ∼50% of children have an episode of wheezing before the age of 6 years and 80–90% of those episodes are triggered by a viral infection.14 Recurrent wheezing impacts ∼22% of children who are of preschool age and can negatively impact quality of life and increased health-care utilization.15 Wheezing in younger children is highly heterogeneous and does not always indicate asthma.16 Other common causes of wheezing in children can include bronchiolitis, tracheomalacia, chronic lung disease, swallowing disorders, and foreign body aspiration.17
Asthma can be difficult to distinguish from wheezing with illness of childhood, asthma predictive indexes have been developed to aid in diagnosing asthma by using recurrent wheeze, response to a bronchodilator, personal history of eczema, aeroallergen sensitivity, eosinophilia, and family history of asthma or allergic rhinitis.10,12 When a child has recurrent wheezing, it is more likely that the child has asthma, therefore, both the NAEPP10 and GINA12 suggest children with ≥3 episodes of wheezing in their lifetime or 2 wheezing episodes triggered by a respiratory tract infection in the past year and a lack of wheezing in between may benefit from a short course (7–10 d) of inhaled corticosteroids (ICS), along with as-needed inhaled short-acting β-agonist (SABA) bronchodilators.10,12 However, there are subtle differences between the NAEPP10 and the GINA12 in the management of intermittent ICS.
NAEPP versus GINA.
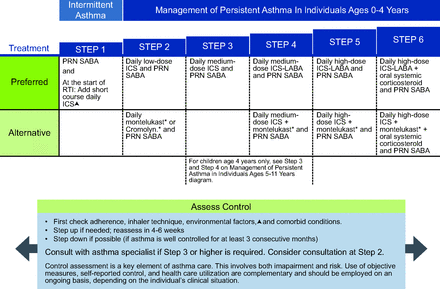
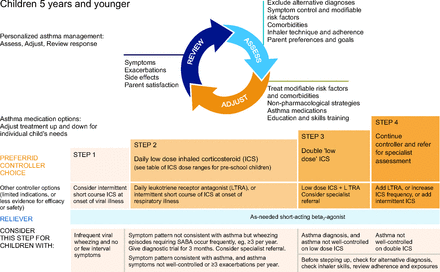
NAEPP10 conditionally recommends a short course (7–10 d) of daily ICS for children ages 0–4 years with recurrent wheeze triggered by a respiratory tract infection (Fig. 1). This recommendation is not continued once the child reaches age 5 years because there is not substantial supportive evidence in older children.10 The GINA12 report similarly recommends considering intermittent high-dose ICS for children ages equal or less than 5 years with intermittent viral induced wheeze and no symptoms between illnesses, but initial episodes of wheezing in children < 1 year can occur in the setting of bronchiolitis and should be managed according to the bronchiolitis guidelines (Fig. 2).
National Asthma Education and Prevention Program (NAEPP) asthma management for individuals ages 0–4 y (triangle symbol). Updated based on the 2020 guidelines. *Montelukast and cromolyn were not considered for this update and/or have limited availability in the United States. The Food and Drug Administration (FDA) issued a boxed warning for montelukast in March 2020. From Reference 10, with permission.
Global Initiative for Asthma (GINA) asthma management for individuals ages ≤ 5 y. From Reference 12, with permission.
Evidence.
Both NAEPP10 and GINA12 based their preferred treatment of younger children on the following studies. Ducharme et al18 showed that starting high-dose fluticasone propionate at the onset of a respiratory tract infection reduced symptom duration and severity, days of SABA use, the frequency of oral steroids, and asthma’s negative effect on quality of life. Kaiser et al19 meta analysis of evidence found high-dose intermittent ICS taken over 7–10 d at the first sign of respiratory tract infection had a 35% risk reduction in severe asthma exacerbations, by decreasing oral steroids use, emergency department visits, and hospitalizations. Bacharier et al20 reported that adding budesonide or montelukast early in respiratory tract infections in addition to SABA did not increase wheezing episode-free days or decrease oral corticosteroid use over 12 months, but the severity of the illness was reduced. Svedmyr et al21 found that adding budesonide at the first sign of a respiratory tract infection reduced cough, noisy breathing, and sleep disturbance, and there were less-severe asthma symptoms; but the study did not show any difference in emergency department visits or hospitalizations.
Choosing the Correct Device for Young Children.
NAEPP9 does not have specific age recommendations regarding specific inhaler devices and use of spacer chamber but do specify that it is important to ensure the caregiver and/or patient can demonstrate good device technique. GINA12 recommends that children ages 0–3 years use a pressurized metered-dose inhaler with a spacer and a face mask and that children ages ≥ 4 years should use a pressurized metered-dose inhaler with a spacer and mouthpiece. Coordination of a pressurized metered-dose inhaler with a spacer and mouthpiece is child dependent and the technique should be assessed before transitioning from the spacer and face mask. Both committees state that nebulizers can be an alternative delivery system but should only be used if a pressurized metered-dose inhaler with a spacer and face mask is not a viable option.9,12 Studies found that children ages < 5 years who are cooperative achieve better medication deposition into the lungs when using a spacer and face mask.22 Every effort should be made to get the child’s cooperation and make inhaled medication delivery easy for the parents and/or caregivers.
Intermittent Maintenance and Relief Therapy
SABA has been the first line of therapy for asthma symptom relief. Studies have shown that adding daily ICS for mild asthma can improve FEV1, FEV1/FVC, and peak expiratory flow, and reduce the need for SABA.23 However, adherence to daily maintenance therapy is an ongoing concern; studies have shown that the rate of nonadherence of daily maintenance therapy in adult and pediatric asthma patients is between 30 and 70%.24 Poor medication adherence is associated with increased health-care cost, lower quality of life, and a greater risk of mortality.25 Common causes for poor medication adherence can include a lack of understanding of the disease, lack of involvement in shared decision-making about daily care, a complex medication regiment, multiple medications to be taken daily, medication costs, and inadequate health literacy.26 Ideally, individuals should be compliant with their medication regiment for the best daily symptom control of asthma; however, there are some individuals who are not willing or able to consistently take daily medications. In this instance, providers may prescribe an intermittent SABA and ICS therapy.
NAEPP versus GINA.
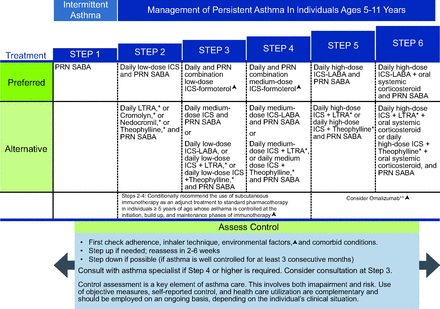
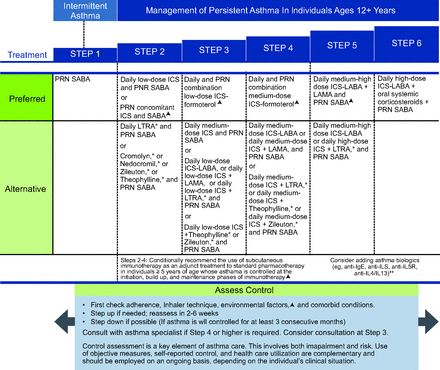
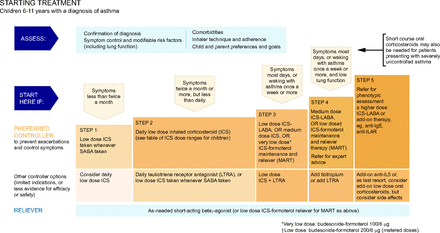
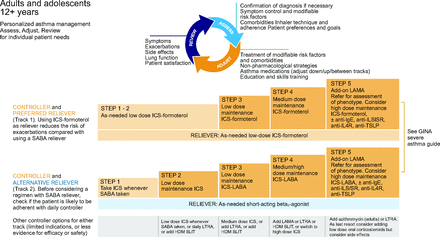
NAEPP10 suggests that daily low-dose ICS is the preferred treatment of choice for mild persistent asthma (step 2) for children ages 5–11 years (Fig. 3). For adolescents (ages ≥12 years) and adults, the NAEPP has 2 preferred treatment choices for mild persistent asthma (Fig. 4). The first is daily low-dose ICS with as-needed SABA and the second is as-needed concomitant ICS and SABA at the first sign of asthma symptoms.10 NAEPP10 suggests individuals who have a low perception of symptoms are more likely to have severe exacerbations and should not use intermittent maintenance therapy and should be placed on a daily maintenance therapy. NAEPP10 did not make recommendations for intermittent ICS for children ages 5–11 years because of the low certainty of evidence, and the 1 published study was difficult to interpret because of the study design.27 The preferred therapy in the GINA is as needed intermittent low-dose ICS to be taken whenever SABA is taken for step 1 in the management of mild asthma in individuals ages 6–11 years (Fig. 5). For 12 years and older the preferred treatment is as needed low dose ICS-formoteral for step 1 and 2 with the alternative treatment option being to take ICS whenever SABA is taken (Fig. 6). One key difference in the asthma severity classification between the NAEPP10 and the GINA12 is that NAEPP10 continues to classify asthma as intermittent and mild, moderate, and severe persistent based on symptom frequency. GINA12 includes mild, moderate, and severe asthma severity classifications, with emphasis that asthma severity should be based on treatment required to control the patients symptoms and exacerbations rather than symptom frequency. The distinction is important because the NAEPP10 still suggests treatment with SABA alone in children ages 5–11 years and older in step 1 (intermittent asthma) and daily preventive ICS and intermittent SABA in step 2 (mild persistent asthma), but GINA12 suggests treating mild intermittent and mild persistent asthma in 12 years and older with the same regiment of only using ICS-formoterol as needed for symptom relief (Fig. 5 and 6).
National Asthma Education and Prevention Program (NAEPP) asthma management for individuals ages 5–11 y (triangle symbol). Updated based on the 2020 guidelines. *Cromolyn, nedocromil, and leukotriene receptor antagonists (LTRA), including montelukast, have an increased risk of adverse consequences and the need for monitoring that make their use less desirable. The Food and Drug Administration (FDA) issued a boxed warning for montelukast in March 2020. From Reference 10, with permission.
National Asthma Education and Prevention Program (NAEPP) asthma management for individuals ages ≥ 12 y (triangle symbol). Updated based on the 2020 guidelines. *Cromolyn, nedocromil, and leukotriene receptor antagonists (LTRA), including zileuton and montelukast, and theophylline were not considered for this update and/or have limited availability for use in the United States, and/or have an increased risk of adverse consequences and need for monitoring that make their use less desirable. The Food and Drug Administration (FDA) issued a boxed warning for montelukast in March 2020. **The Agency for Healthcare Research and Quality (AHRQ) systematic reviews that informed this report did not include studies that examined the role of asthma biologics. Thus, this report does not contain specific recommendations for the use of biologics in asthma in steps 5 and 6. From Reference 10, with permission.
Global Initiative for Asthma (GINA) asthma management for children ages 6–11 y. From Reference 12, with permission.
Global Initiative for Asthma (GINA) asthma management for adolescents and adults. HDM SLIT = house dust mite sublingual immunotherapy. From Reference 12, with permission.
Evidence.
Daily maintenance therapy is associated with better asthma control, but results of studies have indicated intermittent ICS with SABA can be equally effective in reducing asthma exacerbations, severity of symptoms, and quality of life between daily and intermittent ICS users.28-30 There was not any significant difference in peak expiratory flow or FEV1. between daily and intermittent ICS with SABA as needed with symptoms is equally effective in reducing exacerbations, this individualized plan may be used in patients when ICS is not affordable and/or when there are significantly adverse reactions with daily ICS use.28-30
Single Maintenance and Relief Therapy
Single maintenance and relief therapy (SMART) is a new strategy of treating moderate-to-severe asthma by using 1 inhaler for both maintenance and relief. In previous years, standard therapy for moderate-to-severe asthma has been daily medium-dose ICS or low-to-medium ICS with a long-acting β agonist (LABA) and as-needed SABA. Recent studies show that individuals may benefit from a single inhaler for their maintenance and relief therapy for convenience, reduced asthma exacerbation risk, and lower maintenance dosing.31,32 The most important point to remember with SMART is the LABA must contain formoterol. Formoterol has been shown to have a rapid onset of action similar to SABAs to provide the needed rapid relief and because formoterol is the only LABA that currently has evidence supporting effectiveness in SMART.33 It is also worth noting that LABAs without ICS should not be used as a monotherapy in asthma and should be added after failure of ICS monotherapy.34
NAEPP versus GINA.
Both the NAEPP10 and GINA12 have recommendations for single maintenance and relief therapy. The NAEPP10 has SMART as the preferred therapy for children ages 5–11 years (Fig. 3, steps 3 and 4) and individuals ages ≥ 12 years (Fig. 4, steps 3 and 4) with moderate-to-severe asthma. The therapy dosing recommendation for individuals ages 5–11 years and for those ages ≥12 years is low- to medium-dose ICS formoterol to be taken daily and as needed as the preferred therapy.10 NAEPP10 dosing recommendations specify the maximum number of puffs for each age range: 8 puffs for ages 5–11 years and 12 puffs for ages ≥ 12 years in a 24-h. NAEPP10 suggests alternate therapy for moderate-to-severe asthma in children ages 5–11-year (Fig. 3, steps 3 and 4) can be daily medium-dose ICS or ICS-LABA with as-needed SABA and for those ages ≥ 12 years (Fig. 4, steps 3 and 4) medium dose ICS, ICS-LABA, or ICS plus long-acting muscarinic antagonist with as-needed SABA.10
If the individual’s asthma severity increases to severe per the NAEPP,10 the preferred relief therapy changes from ICS-formoterol to as-needed SABA. Specifically, in children ages 5–11 years, if controller medication escalation is required to daily high-dose ICS-LABA in steps 5 and 6, then the NAEPP10 recommends returning to SABA as needed for relief (instead of ICS-formoterol) or adding oral systemic steroids with as-needed SABA (Fig. 3, steps 5 and 6). Similarly, in individuals ages ≥ 12 years, in step 5 and 6, when increasing from medium- to high-dose ICS-LABA adding a long-acting muscarinic antagonist or systemic oral steroids. NAEPP10 recommends as-needed SABA for relief (not ICS-formoterol) (Fig. 4, steps 5 and 6).10 The low, medium, and high dosing of ICS differs slightly between NAEPP and GINA and may different due to different formulations available in the US versus global market. NAEPP9 ICS dosing recommendations are based on the age range of the individual but GINA12 dosing recommendations are for 6 years and older. The NAEPP7 Expert Panel recommends that the dosing definitions need to be updated.10
GINA12 has SMART as one of the preferred treatment choices for children ages 6–11 years with moderate asthma (Fig. 5, step 3) and for individuals ages ≥ 12 years (Fig. 6, steps 1–5). The GINA12 maximum recommended daily dose of the as-needed ICS-formoterol should not exceed 72 µg of formoterol. NAEPP10 states that if an individual is well controlled on current maintenance and relief therapy, then they do not recommend making adjustments to individual mediation regiment to align with updated guidelines. GINA12 recommendations have 2 separate reliever treatment tracks for individuals ages ≥12 years (Fig. 6). In track 1, the reliever is as-needed low-dose ICS-formoterol, and, in track 2, it is as-needed SABA.12
GINA12 recommends assessment of a individuals average frequency of ICS-formoterol use to the 4 weeks as a way to determine further escalation of controller therapy as needed. Track 2 of the GINA offers a separate alternative option of using SABA as a reliever in steps 1–5, similar to the NAEPP Expert Panel Report 3 guidelines18 (from 2007) for individuals ages ≥ 12 years when using daily ICS maintenance alone, or ICS in combination with a LABA other than formoterol. In the United States, this second option may be important when users do not tolerate LABAs due to adverse effects or when insurance preferred medications that include salmeterol or vilanterol, which are not currently recommended for SMART. For individuals ages ≥ 12 years with severe asthma (Fig. 4, step 5), NAEPP10 newly recommends to add a long-acting muscarinic antagonist and consider high-dose ICS-formoterol.12 GINA12 states that beclomethasone-formoterol may be a suitable ICS-formoterol option, although this is left out of the NAEPP,10 likely because it is not available in the United States.
Evidence.
Both GINA12 and NAEPP10 recommend using only ICS-formoterol as SMART because formoterol has a rapid onset and long duration of action.33,35 Many large well-designed studies that used budesonide-formoterol as SMART has shown a decrease in frequency, severity, and duration of asthma exacerbations, maintained daily asthma control, improved lung function and less need of an overall ICS dose.31,36-41
Exercise Pretreatment with Single Maintenance and Relief Therapy.
Nearly 90% of people with asthma experience exercise-induced bronchospasms.42 The best way to prevent asthma symptoms during or after exercise is to pretreat with reliever therapy. GINA12 suggests that ICS-formoterol can be used for pretreatment before exercising, in referencing one 6-week study in which ICS-formoterol has similar results to low-dose ICS with as-needed SABA,43 but acknowledges that more studies are needed. NAEPP,10 however, does not address which medication to use as a pretreatment before exercise when an individual is using SMART.
Considerations and Limitations.
It is important for respiratory therapists and other health-care providers to be aware of current limitations with the implementation of SMART in the United States The United States Food and Drug Administration has not approved ICS-formoterol to be used more often than twice a day or for acute relief. The lack of response from the Food and Drug Administration with regard to the recommended shift to the use of ICS-formoterol as a reliever medication may reduce incorporation of SMART as an asthma standard of care in the United States because insurers may not reimburse for the medication. Medicare and other payers preferred drug lists are typically derived by medically approved medications that are cost-effective.
Studies that looked at ICS-formoterol were done with dry powder inhalers currently not available in the United States, so there may be some questions as to whether the United States–based drugs and delivery devices produce the same efficacy.38 Drug costs with ICS-formoterol are currently more expensive than SABAs, and payers may not consider them to be cost-effective. There are insurance dispensing limitations of controller medications typically to one a month. If an individual’s asthma is poorly controlled and he or she has been prescribed SMART, then there could be risk of him or her running out of the medication before a refill can be dispensed. The intermittent ICS and SMART therapy asthma management strategies are significantly different in the past few decades and many result in conflicting information being given to patients and families. It will be important that respiratory therapists, and other healthcare professionals educated on the new NAEPP10 and GINA12 to minimize conflicting information and confusion of patients and families.
Short-Term Increase in Daily ICS
ICS are typically prescribed twice daily as maintenance medications for asthma. Previous studies showed a benefit in doubling, tripling, or quadrupling the regular daily ICS dosing during an asthma exacerbation.44-46 Based on these studies, a short-term increase in ICS during an exacerbation was integrated into national and global guiding documents for asthma.7,8,12 As more studies are done, there is less noted benefit on a short-term increase in ICS during an asthma exacerbation.
NAEPP versus GINA.
NAEPP4,10 does not recommend a temporary increase in ICS for worsening asthma symptoms in individuals ages 4–11 years who are likely to adhere to their daily medication because recent studies did not observe improved quality of life or a statistically significant reduction in the rate of exacerbation, hospitalizations, in patients temporarily increasing ICS therapy compared to controls. NAEPP10 states that clinicians can consider quadrupling the regular dose at the first sign of illness for individuals ages ≥ 16 years whose adherence is questionable but it is not recommended if the individual is compliant with daily medications. GINA12 recommendations, state that a short-term increase in maintenance ICS dose for 1–2 weeks may be necessary and can be initiated according to its asthma action plan. Both NAEPP10 and GINA12 state that the best treatment of choice in preventing asthma exacerbations is a daily maintenance medication.
Evidence.
Jackson et al47 showed children 5-11 years of age with mild to moderate asthma who increased their daily maintenance meditation at the first sign of asthma symptoms did not have statistically significant reduction in rate of severe asthma exacerbations or improved health outcomes, but those patients had a 0.23 cm per year decreased linear growth (P = .6), McKeever et al48 showed adolescents (16 years and older) and adults who received a temporary quadrupled ICS dose at the onset of asthma symptoms had fewer severe asthma exacerbations, but there was concern with daily medication adherence in ∼50% of the study participants, which makes it difficult to determine if quadrupling the medication was effective if one was truly compliant. The NAEPP10 systematic review did not find a significant reduction in asthma exacerbations and hospitalizations in adolescent and adults.10,44,46,49
Long-Acting Muscarinic Antagonists
Asthma is caused by airway inflammation, hyersecretion, and smooth muscle contraction.50 The vagal nerve is the parasympathetic nervous system has acetylcholine neurotransmitters in the submucosal glands, smooth muscle, and epithelial calls throughout the bronchial tree that incluces bronchoconstriction and mucus secretion.50 Long acting muscurinic antagonists block acetylcholine which prevents vagal nerve induced reflex bronchocontriction and mucas secretions.50 Long acting muscurinic therapy is sometimes recommended as add-on therapy for asthma control.
NAEPP versus GINA.
NAEPP10 conditionally recommends against adding a long-acting muscarinic antagonist (LAMA) to ICS therapy when compared with ICS-LABA in individuals ages ≥ 12 years and with uncontrolled asthma. NAEPP10 suggests the LABA benefit to harm was more favorable than LAMA. NAEPP10 did conditionally recommend adding a LAMA to ICS in patients ages ≥ 12 years if they were not able to tolerate LABA and were not well controlled on ICS alone.10 GINA12 states LAMA may be considered as add-on therapy in individuals ages 12 years and older, but not to be used in children 6 to 11 years of age. LAMA can be used as a triple combination inhaler therapy for individuals ages ≥ 18 years if asthma is poorly controlled and on at least medium- to high-dose ICS-LABA. GINA8 does not specifically address whether LABAs are preferred add on therapy over LAMAs when someone is not controlled with asthma alone. GINA8 recommends increasing ICS-LABA to at least medium dose before adding a LAMA and does not commend adding a LAMA for patients who have persistent dyspnea. Both NAEPP10 and GINA12 do not recommend LAMA being used in asthma management without an ICS.
Evidence.
May be a useful add-on therapy in poorly controlled asthma with ICS monotherapy because it reduces air-flow obstruction and improves symptoms and lung function in moderate persistent asthma in school age children to adults.51-55 Adding a once-daily long-acting muscarinic antagonist to ICS-LABA either as a standalone or as a triple medication has been found to improve lung function, reduce the need for oral steroids, and modestly reduce exacerbations in poorly controlled moderate-to-severe asthma with medium- to high-dose ICS or ICS-LABA.56-59 Two separate studies found that adding LABA-formoterol to ICS improved FEV1, and decreased asthma symptoms as well as oral steroid and reliever medication use faster and better compared with tiotropium (long-acting muscarinic antagonist) or other second-line asthma regiment (methylxanthine or leukotriene modifier).60,61 These studies support the addition of long-acting muscarinic antagonist to ICS-LABA or ICS monotherapy in patients poorly controlled with these therapies, although ICS–long-acting muscarinic antagonist may be inferior to LABA-ICS.
Exhaled Nitric Oxide
Fraction of exhaled nitric oxide ( FENO) levels in gas form have been used as a noninvasive way to assess and monitor airway inflammation in asthma and other pulmonary diseases. Higher levels of FENO (>50 ppb in adults and >35 ppb in children ages 5–12 years) in nonsmokers is moderately associated with eosinophilic airway inflammation.10,62 The measurement of FENO has become a standard of care in assessing and monitoring airway inflammation, response to ICS therapy, and medication adherence to daily maintenance therapy.63-65
NAEPP versus GINA.
NAEPP10 states that FENO in isolation is not useful for an asthma diagnosis in patients ages > 5 years or in young children to predict the future development of asthma. FENO should not be used in isolation, rather as an adjunct to determine the need for increasing therapy or adding anti-inflammatory therapy.10 GINA12 recognizes that FENO is not widely available for most children. An FENO measurement in a single point of time should be interpreted with caution.66
Evidence.
Pulmonary function testing can identify airflow limitations and reversibility with bronchiolar administration or hyperactive airway response with bronchial provocation.67 FENO can further assist in detecting eosinophilic airway inflammation, corticosteroid responsiveness, ongoing monitoring of airway inflammation, and, potentially, poor medication adherence.68 The study done by Garg et al69 attempted to increase ICS dose in response to elevated FENO but did not find any statistically significant reduction in ICS or exacerbations in the targeted therapy. Whereas the study by Honkoop et al70 found that targeting FENO less than 25 ppb and a symptom-free strategy reduced medication and improved quality of life but did not decrease severe asthma exacerbation rates. Lower FENO levels were associated with improved asthma control, small airway function, and a lower steroid dose in children.71,72 Stepping up medications based on FENO levels significantly decreased the number of exacerbations and FENO levels but did not impact day-to-day clinical symptoms and, in some incidences, resulted in higher ICS dosing without improvement in symptoms.73-75 Other studies found that elevated FENO is a poor marker for asthma control in children who report consistent use of their daily medication.76 The conflicting information supports why assessing FENO levels should not be used as a standalone diagnostic procedure in asthma management.
Indoor Allergen Mitigation
Control of environmental factors is an essential part of asthma management. Indoor allergens are associated with an increase in asthma symptoms and asthma exacerbations.77 Common indoor allergens include dust mites, pet dander, molds, and pest feces.77,78 Allergen mitigation interventions decrease exposure to the known allergen and can be single or multicomponent. A single intervention is one single strategy to target one or more specific allergens.10 A multicomponent intervention is ≥ 2 single interventions as part of a bundle approach.
NAEPP versus GINA.
NAEPP10 recommends against allergen mitigation in individuals with asthma who do not have sensitization to a specific indoor allergen or who do not have symptoms related to the indoor allergy exposure. Sensitization is defined as a production of immunoglobulin E to an aeroallergen confirmed by a skin test or a serum assay for a specific immunoglobulin E.10 If an individual has symptoms with exposure to indoor allergens, then NAEPP7 conditionally recommends a multicomponent mitigation intervention. It also conditionally recommends that an individual with pest sensitization use integrated pest management as an individual or multicomponent mitigation intervention; specifically, NAEPP10 recommends for dust mites to use impermeable pillow and mattress covers only as a part of multicomponent mitigation intervention, not as a single component. GINA12 does not recommend allergen avoidance as a general strategy, and, for individuals who exhibit sensitivity to an allergen, GINA12 indicates that there is limited evidence of clinical benefit in a single-component strategy. GINA12 states to consider a trial of simple mitigation strategies but cost should be part of the consideration.
Evidence.
Studies assessed the effectiveness of various indoor allergy mitigation interventions. Both the NAEPP10 and GINA12 committees state that most of the studies were inconclusive. Single interventions were not associated with improved asthma control, exacerbations, health-care utilization, and quality of life.79 Multicomponent interventions with an environmental focus can improve quality of life and productivity.80,81 Crocker et al81 found multicomponent environmental interventions to be helpful in children and adolescents with asthma, but the same effectiveness was inconclusive in adults.
Immunotherapy
Allergic rhinitis affects up to 40% of Americans and is common in individuals with allergic asthma.82,83 Allergic asthma is one of the most common forms of asthma and is defined as having symptoms after an acute exposure to an allergen or a specific season.84 Immunotherapy (allergy shots) is a preventive therapy that incorporates incremental high-dose exposure to a known allergen over time, reducing immunoglobulin E–mediated allergic clinical response to those allergens.85 Immunotherapy can be administered subcutaneously or sublingually.86
NAEPP versus GINA.
NAEPP10 conditionally recommends the use of subcutaneous allergen immunotherapy as an adjunct therapy for children ages ≥ 5 years who have demonstrated allergic sensitization or worsening asthma symptoms in mild-to-moderate allergic asthma that is well controlled at the initiation, buildup, or maintenance phase of immunotherapy but should not be used in severe asthma. NAEPP,10 however, does not support the use of sublingual immunotherapy for the treatment of asthma. GINA12 recommends allergen-specific immunotherapy as a treatment option if the allergen plays a role in allergic rhinoconjunctivitis.
Evidence.
A Cochrane review found that European physicians tend to favor single-allergen immunotherapy compared with North American physicians who prescribe multiple-allergens treatments.87 Few studies compared immunotherapy with pharmacology therapy, comparing asthma exacerbations, asthma control, and quality of life. There have been no studies that assessed the impact of immunotherapy on emergency department visits, clinic visits, or hospitalizations. Several studies showed insignificant benefit of immunotherapy to asthma exacerbations and asthma control but did have a positive impact on quality of life.88-95 Subcutaneous immunotherapy can be helpful for asthma control but does have a risk of anaphylaxis.87 Fatal or near anaphylaxis rates varied greatly with subcutaneous immunotherapy and should be used with caution.87,96 Sublingual immunotherapy may reduce quick-relief medication use and may reduce long-term medication use.97-100 There are only certain forms of sublingual immunotherapy currently approved by the Food and Drug Administration and liquid sublingual immunotherapy is not Food and Drug Administration approved and is used off label in the United States.101
Bronchial Thermoplasty
Bronchial thermoplasty is a relatively new bronchoscopy treatment for adults with moderate-to-severe asthma whose asthma symptoms remain poorly controlled despite optimal medical therapy. The bronchoscopy treatment delivers local radiofrequency energy to the large airways, which causes airway remodeling by reducing airway smooth muscle and by modulating the composition of an extracellular matrix.102
NAEPP versus GINA.
NAEPP10 does not recommended bronchial thermoplasty as a treatment option for persistent asthma because the small benefit does not outweigh the risks (eg, infection, hemoptysis, bronchiectasis, atelectasis). Some individuals still opt for this treatment. GINA12 similarly states that bronchial thermoplasty is a potential treatment option for adults whose asthma regiment. uncontrolled, despite optimal therapy regiment. Both NAEPP10 and GINA12 also state that bronchial thermoplasty should be done by a trained and experienced specialist in an appropriate treatment center and after comorbidities have been addressed and medication adherence has been optimized.
Evidence.
Bronchial thermoplasty has not been well studied in individuals ages < 18 years and was found to have a large placebo effect.103 The placebo effect is defined as a beneficial health outcome that results from an individual’s anticipating the intervention will help.104 Castro et al103 found that adults taking high-dose ICS-LABA had an increase in asthma exacerbations the first 3 months, and, over time, there was a sustained decrease in exacerbations compared with pretreatment. There was no noted improvement in lung function after bronchial thermoplasty and long-term benefit is not known due to the lack of studies beyond 5 years.103,105
COVID-19 and Asthma
SARS-CoV-2 was the cause of the acute respiratory COVID-19 pandemic. COVID-19 was found to spread by aerosolization through coughing, sneezing, and/or speaking.106 Those individuals severely affected by COVID-19 present with dry cough, fever, fatigue, loss of taste and smell, and shortness of breath that can develop into pneumonia and ARDS.107 Respiratory viruses are a common trigger for asthma and lead to frequent hospitalizations, emergency department visits, and high mortality.108 Over the past two years, individuals with well controlled mild to moderate asthma have no increased risk of acquiring COVID-19 or experiencing more severe symptoms from COVID-19.109 The increased risk of COVID-19 related hospitalizations and motility with asthma was largely associated with age and asthma related comorbidities (cardiovascular disease, diabetes).109
NAEPP versus GINA.
The recent NAEPP10 did not address asthma management in the face of COVID-19. GINA12 continues to reinforce the importance that individuals with asthma to have a written asthma action plan and encourages them to continue taking prescribed medications, particularly ICS. GINA12 also recommends avoiding spirometry and use of nebulizers when COVID-19 is confirmed, suspected, or local risk is moderate to high, to minimize the risk of aerosolization and transmission of the virus; and follow infection control recommendations; and it recommends receiving the COVID-19 vaccine.
Evidence.
Several studies found that asthma did not place an individual at greater risk of acquiring COVID-19, and systematic reviews have not shown an increase in risk of having a more severe case of COVID-19 if contracted.110,111 It was identified that there was a risk of COVID-19–related death for people who had poorly controlled asthma, specifically those who required recent oral corticosteroids for their asthma.104
Summary
Both NAEPP10 and GINA12 recommendations are valuable resources used to improve asthma management clinical practice guidelines for all ages in the United States. There are conflicting recommendations between the two asthma management resources on most of the guidelines reviewed in this narrative. Key stakeholders should come to a consensus on the best asthma management resource to follow within one’s designated organization. Once the clinical practice changes have been determined, the stakeholders should identify and address barriers that prevent incorporation of the suggested asthma management practice changes.
This may include discussions with local health insurance payers, pharmacies, and pharmaceutical companies in addressing reimbursement and access to medications. Educational content for frontline health-care providers and prescribing providers should be developed by using multiple aspects of communication about the practices changes and why. Patient education materials will likely need to be modified, including the electronic or printed versions of an asthma action plan, to incorporate intermittent ICS, concomitant maintenance and relief therapy, and SMART. Health-care providers need to review the NAEPP10 and GINA12 as they continue to be revised and to incorporate shared decision making into discussions with individuals about their asthma plan. Although there are differences between the NAEPP10 and GINA12 the increased therapeutic options for providers will allow improved individualized care of each patient with asthma.
Footnotes
- Correspondence: Joyce A Baker MBA RRT RRT-NPS AE-C FAARC, Breathing Institute, Children’s Hospital Colorado, 13123 E 16th Ave, Aurora, CO 80045. E-mail: joyce.baker{at}childrenscolorado.org
The authors have disclosed no conflicts of interest.
- Copyright © 2023 by Daedalus Enterprises
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.
- 38.↵
- 39.
- 40.
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.
- 53.
- 54.
- 55.↵
- 56.↵
- 57.
- 58.
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.
- 90.
- 91.
- 92.
- 93.
- 94.
- 95.↵
- 96.↵
- 97.↵
- 98.
- 99.
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵