Abstract
BACKGROUND: Peak flow testing is a common procedure performed in ambulatory care. There are currently no data regarding aerosol generation during this procedure. Given the ongoing debate regarding the potential for aerosol transmission of SARS-CoV-2, we aimed to quantify and characterize aerosol generation during peak flow testing.
METHODS: Five healthy volunteers performed peak flow maneuvers in a particle-free laboratory space. Two devices continuously sampled the ambient air during the procedure. One device can detect ultrafine particles 0.02–1 μm in diameter, while the second device can detect particles 0.3, 0.5, 1.0, 2.0, 5.0, and 10 μm in diameter. Five different peak flow meters were compared to ambient baseline during masked and unmasked tidal breathing.
RESULTS: Ultrafine particles (0.02–1 μm) were generated during peak flow measurement. There was no significant difference in ultrafine particle mean concentration between peak flow meters (P = .23): Respironics (1.25 ± 0.47 particles/mL), Philips (3.06 ± 1.22), Clement Clarke (3.55 ± 1.22 particles/mL), Respironics Low Range (3.50 ± 1.52 particles/mL), and Monaghan (3.78 ± 1.31 particles/mL). Ultrafine particle mean concentration with peak flow testing was significantly higher than masked (0.22 ± 0.29 particles/mL) and unmasked tidal breathing (0.15 ± 0.18 particles/mL, P = .01), but the ultrafine particle concentrations were small compared to ambient particle concentrations in a pulmonary function testing room (89.9 ± 8.95 particles/mL).
CONCLUSIONS: In this study, aerosol generation was present during peak flow testing, but concentrations were small compared to the background particle concentration in the ambient clinical environment. Surgical masks and eye protection are likely sufficient infection control measures during peak expiratory flow testing in asymptomatic patients with well controlled respiratory symptoms, but COVID-19 testing remains prudent in patients with acute respiratory symptoms prior to evaluation and peak expiratory flow assessment while the community prevalence of SARS-CoV-2 cases remains high.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus causing the COVID-19 global pandemic, is spread through various modes of transmission, including direct contact with an infected individual, indirect contact with contaminated surfaces, aerosol generation during procedures such as endotracheal intubation or bronchoscopy, and droplet transmission, which is felt to be the primary mode of transmission.1 Droplet particles are typically characterized as larger than 5–10 µm, originating from the upper airway and expelled during expiratory activities such as breathing, talking, coughing, and sneezing. Droplet transmission is felt to occur primarily within 6 feet of distance, as the larger particles tend to settle quickly on nearby surfaces.1,2 However, evidence suggests smaller droplet particles are able to spread over much greater distances.3-5 Particles smaller than 5–10 µm have been defined as “aerosols” or “droplet nuclei” and can remain airborne for extended periods of time, traveling greater distances, and can cause transmission by settling into the lower respiratory tract.6,7
There is a growing body of evidence that SARS-CoV-2 may have the potential to spread via airborne transmission.8-16 Multiple clinical studies have reported that SARS-CoV-2 RNA can be detected in air samples surrounding infected individuals, but these studies were unable to document viable virus.17-20 However, Fears et al21 reported that aerosolized SARS-CoV-2 retained infectivity and virion integrity for up to 16 h. Additionally, both Morawska et al22 and Papineni et al6 demonstrated that the majority of particles generated during a variety of expiratory activities (including normal tidal breathing and speaking) were < 1 µm. Alsved et al23 documented increasing aerosol particle generation between breathing, talking, singing, and loud singing, respectively, suggesting that more intense expiratory maneuvers produce higher concentrations of small particles.
Given the evidence of aerosol particle generation during expiratory activities and the potential for aerosol transmission of SARS-CoV-2, we were interested in better understanding particle generation during peak expiratory flow (PEF) testing. Performed commonly in the out-patient setting in the offices of primary care providers, pulmonologists, and allergists, PEF testing is characterized by intense expiration, potentially posing infectious risk to health care workers administering this procedure and other individuals in close proximity. Our aim was to compare small particle generation between various PEF devices used within our Mayo Clinic Enterprise Healthcare System, using a pragmatic approach, to inform our infection control recommendations and practice during the ongoing pandemic. Additionally, we sought to further characterize particle generation by quantifying particle size, as there is evidence that smaller particles are more likely to remain airborne for an extended duration, potentially conferring increased infectious risk.
Quick Look
Current Knowledge
Peak flow testing is a common procedure performed in ambulatory settings. Aerosol and droplet generation associated with peak flow testing may have significant infection control and safety implications during the COVID-19 pandemic.
What This Paper Contributes to Our Knowledge
We developed a pragmatic approach to measure small particle generation after peak flow testing in a particle-free environment. Different peak flow meters were associated with similar increases in small particle generation, but quantities were very small compared to particle concentrations commonly found in the ambient clinical environment.
Methods
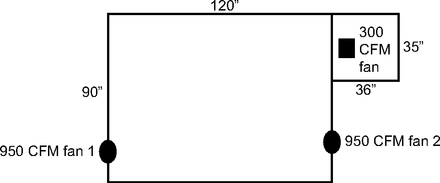
A prospective study was performed with 5 healthy volunteers. To optimize signal-to-noise ratio and to detect ultrafine particle generation in the ambient air, the study was performed in a highly controlled, sealed, nearly particle-free room the size of a body plethysmograph box (74 × 36 × 35 inches, 56 ft3, 1,570 L). This sealed room was separated from a larger sealed space (74 × 90 × 120 inches, 463 ft3, 13,110 L) by a nearly airtight door. The entire experimental space was connected in series to 2 portable 950 CFM fans with HEPA filtration model H1000V (Abatement Technologies, Suwanee, Georgia), which allowed particle concentration of the experimental space to be reduced to < 1 particle per cubic centimeter prior to each subject testing series (Fig. 1). Air flow was switched off during the tests. Ambient baseline air was sampled prior to peak flow measurement while the volunteer breathed quietly with and without a type 2 procedural mask. Continuous particle detection was performed during 3 sequential PEF measurements without a mask using each device, using 2 particle counters: an ultrafine particle counter, PTrak 8525 (TSI, Shoreview, Minnesota) for particles 0.02–1 µm in diameter and Fluke 985 (Fluke Corporation, Everett, Washington) for particles 0.3, 0.5, 1.0, 2.0, 5.0, and 10 µm in diameter. These sensors were positioned 12 inches in front of each subject. A HEPA filter recirculator (ASA model #SS-300-PFS, Sentry Air System, Houston, Texas) was placed in the small sealed room underneath the seated subject’s chair and was used to return particle counts in this space to baseline after each set of PEF meter measurements.
Experimental setup of particle-free laboratory space.
Study Protocol
The study was reviewed and approved by the Mayo Clinic Institutional Review Board (20-006779). Five healthy volunteers performed 5 min of tidal breathing wearing a type 2 procedural mask (ie, masked tidal breathing), then 1 min of unmasked tidal breathing, and then 3 forced expiration maneuvers with each of the 5 peak flow meters listed below.
Peak flow testing was performed in accordance with each device’s manufacturer specifications and recommendations. The following peak flow meters were tested: Respironics peak flow meter and Respironics Low Range peak flow meter (Respironics, Murrysville, Pennsylvania), Philips peak flow meter (Philips, Murrysville, Pennsylvania), Clement Clarke peak flow meter (Haag-Streit Group, Harlow, Essex, United Kingdom), and Monaghan peak flow meter (Monaghan Medical, Plattsville, New York) (Table 1).
Characteristics of Peak Flow Meters
Baseline particle measurements using both the PTrak and the Fluke devices were also performed in a Mayo Clinic Pulmonary Function Laboratory procedure room with 15 air exchanges per hour to provide a baseline reference value for a representative clinical testing environment.
Statistical Analysis
Particle concentration values were captured at 1-s intervals throughout each testing protocol. For graphical clarity, data were smoothed via 5–7-s averaging of the concentration gradient using GraphPad Prism 8.2 (San Diego, California). Given that peak concentrations were highest near the end of peak flow testing, instantaneous particle concentrations were averaged over the final minute of testing to estimate maximum particle concentration. The Friedman test was used to assess for any differences between peak flow meters and to compare peak flow meter particle concentrations to masked and unmasked tidal breathing.
Results
Mean cumulative particle counts as measured with the PTrak device (ie, the ultrafine particle counter) during each step of the peak flow examination with 5 devices, as well as during masked and unmasked tidal breathing, are demonstrated in Figure 2. The mean particle concentration of ultrafine particles ranging from 0.02 to 1 µm (measured in particles/mL) increased after peak flow testing as compared to concentrations during masked (0.22 ± 0.29 particles/mL) and unmasked (0.15 ± 0.18) tidal breathing (P = .01). Mean particle concentration was lowest with the Respironics peak flow meter (1.25 ± 0.47), and were similar between the Philips (3.06 ± 1.22), Clement Clarke (3.55 ± 1.50), Respironics Low Range (3.50 ± 1.53), and Monaghan (3.78 ± 1.31) peak flow meters. However, when comparing ultrafine particle generation between all peak flow meters using the Friedman test, there was no significant difference (P = .23).
Comparison of mean cumulative particle concentrations as measured with the PTrak particle counter detecting particle sizes 0.02–1 µm after 5 healthy subjects performed 3 forced expiratory maneuvers with each peak flow meter.
The mean cumulative particle concentration as measured with the Fluke device (particles 0.3–10 µm in diameter, measured in particles/mL) was higher after peak flow testing compared to masked or unmasked tidal breathing; these values were also similar across the different peak flow meters (Fig. 3). However, smaller particles (0.3 and 0.5 µm) were highest with the Monaghan peak flow meter (0.16 ± 0.15 and 0.07 ± 0.07, respectively). The mean cumulative particle concentrations for larger particles (1.0, 2.0, 5.0, and 10.0 µm) were low (0.001–0.027) and largely similar between peak flow meters (Fig. 3).
Comparison of mean cumulative particle concentrations of different sized particles (0.3–10 µm) as measured with the Fluke 985 counter after 5 healthy subjects performed 3 forced expiratory maneuvers with each peak flow meter.
The mean concentration of ultrafine particles (PTrak, 0.02–1 µm) in the pulmonary function test procedure room was 89.9 ± 8.95 particles/mL. The mean concentration of larger particles (1.0, 2.0, 5.0, and 10.0 µm) was lower that of than ultrafine particles (0.3, 0.5 µm) during peak flow testing (0.014 ± 0.006, 0.020 ± 0.006, 0.004 ± 0.003, and 0.008 ± 0.005 particles/mL vs 0.473 ± 0.033 and 0.054 ± 0.009, respectively).
Discussion
In this study, we quantified and compared small particle generation using a variety of commercially available peak flow meters utilized in our health care system. We were particularly interested in peak flow testing because it is a common procedure widely performed in medical offices, examination rooms, and in patient homes, and there is a paucity of information available regarding the risk of aerosol generation in this context. Given the growing evidence for aerosol production during common pulmonary testing activities and the ongoing debate regarding the risk for aerosol transmission of SARS-CoV-2, assessing the impact of peak flow testing was a priority to inform our infection control recommendations and practice during the ongoing COVID-19 pandemic.4,24,25
The infectious risk of PEF and other pulmonary testing procedures depends on a number of factors. The pretest probability of a given patient being infected with COVID-19 when they present for testing is based on the current community incidence of infections, patient compliance (or ability to comply) with recommended preventive measures, and patient symptoms. Many patients undergoing PEF testing have chronic respiratory symptoms, and in our clinical experience it is challenging to differentiate acute on chronic respiratory complaints due to superimposed COVID-19 infection. Some centers have chosen to perform universal SARS-CoV-2 testing prior to pulmonary procedures, which becomes increasingly important with worsening local community spread of COVID-19.
A number of factors affect the volume and other characteristics of the particle cloud generated when an individual forcibly exhales, including but not limited to the surrounding temperature and humidity, and the size, physical features, and air exchange characteristics of the room in which PEF maneuvers are performed.4,24,25 All these factors must be considered to determine whether a PEF maneuver could pose significant potential risk to health care workers administering the examination or to other individuals nearby. If high quantities of small particles (≤ 5 µm in size) likely to aerosolize were produced (ie, much higher than the particle counts in the ambient environment), this would suggest that airborne precautions should be used during the procedure. Airborne precautions would involve the use of a fit-tested N95 mask or powered air-purifying respirator by health care workers administering the PEF test, in addition to protective eyewear. An understanding of the ventilation in the clinical testing environment is also paramount, as the number of air exchanges per hour would determine when the room could be used again safely. In fact, very high quantities of aerosol production during PEF could argue against performing the procedure in an out-patient clinical environment regardless of infection control practices. Alternatively, low levels of particle generation would favor ongoing use of droplet precautions (eg, surgical mask with eye protection) and may suggest that performing PEF in offices is likely safe if performed with appropriate safety precautions.
Although all peak flow meters measured increased mean particle concentrations compared to masked and unmasked tidal breathing, the differences were small when compared to the mean particle concentrations found in the ambient clinical environment. Overall, the mean concentration of ultrafine particles (0.02–1 µm using the PTrak) was higher than the mean concentration of larger particles (5–10 µm using the Fluke) with all 5 peak flow meters used in this study. This observation may be due to the tendency of smaller particles to remain airborne longer, while larger particles are more likely to fall quickly and settle on surrounding surfaces.
The Respironics peak flow meter had the lowest mean ultrafine particle concentration; however, this was not statistically significant and is unlikely to be clinically important given the much higher particle concentrations already present in the ambient environment. In fact, the small degree of increased particle generation by all peak flow meters necessitated the use of a very small, nearly aerosol-clean environment, as these differences would be difficult to detect in a traditional clinical environment or larger space.
These findings have important implications during the COVID-19 pandemic given the continued concern for SARS-CoV-2 airborne transmission during medical procedures.4,8-14,17-21 The particle characteristics and concentration threshold that may increase the risk of SARS-CoV-2 transmission during various procedures, including peak flow and pulmonary function testing, are not clearly established. Given the small relative increase in particle generation recorded in this study, we have transitioned our infection control practices to droplet precautions with enhanced cleaning protocols for individuals with well controlled respiratory symptoms performing peak flow testing as part of their routine evaluation. We made this decision after careful consideration of the physical characteristics of our clinic space, and after maximizing the ambient air exchanges in all our procedural areas (currently 11–15 air exchanges per hour). Individuals with acute worsening of respiratory symptoms are either managed through virtual care or routinely tested with SARS-CoV-2 PCR prior to an in-person office visit to reduce the pretest probability and subsequent risk of transmission during the evaluation and procedure. The rapidly growing practice of virtual care during the ongoing COVID pandemic underlines the importance of early peak flow meter provision and training in patients with a new diagnosis of asthma in accordance with the Global Initiative for Asthma guidelines, in combination with a clear and complete asthma action plan, to facilitate safe and effective remote management (https://ginasthma.org, Accessed January 4, 2021).
We believe these data are the first to directly measure and characterize aerosol and droplet production during peak flow testing, there are multiple studies that have evaluated particle generation during expiratory activities or medical procedures that share similar findings. Morawska et al22 noted high ambient particle concentrations, which necessitated an experimental design involving a custom-made fan and HEPA filter to reduce particle background and “obtain meaningful measurements of aerosol size distribution.” In our study, unmasked tidal breathing generated 0.15 particles/mL. This is consistent with prior reports of an average concentrations of 0.10–0.17 particles/mL with unmasked breathing.22,26 Additionally, our finding that particles < 1 µm predominated was also consistent with multiple prior studies. For example, Shao et al26 reported that only 0.2% of particles generated during normal breathing were > 5 µm, whereas Yang et al2 reported that 82% of particles produced during coughing ranged from 0.72 to 2.12 µm. Asadi et al27 also observed that the vast majority of particles occurring with various expiratory activities (including breathing, talking, coughing, and jaw movement) were < 5 µm, and that use of a mask shifted particle distribution toward smaller particles. In addition, our data indicated that particle generation during peak flow testing was about an order of magnitude higher than tidal breathing with or without a mask. Similarly, Alsved et al23 reported that loud singing and loud singing with exaggerated diction generated a particle emission rate (in particles/s) about an order of magnitude greater than unmasked tidal breathing, suggesting a similar increase with intense expiratory activity.
This study adds to previous work conducted by our group evaluating the risk of aerosol generation during cardiopulmonary exercise training and during spirometry. Helgeson et al28 reported that light-to-intense exercise while wearing a mask did not generate additional particles beyond what is observed in the ambient clinical environment, but that very intense exercise generated a significant increase in aerosols and warrants additional personal protective measures to mitigate infectious risk. In another study Helgeson and colleagues29 observed that aerosol particles are generated during routine spirometry, warranting additional precautions to mitigate the risk the transmission of SARS-CoV-2. Both studies used the Fluke particle counter and indicated a predominance of particles < 1 µm.
An interesting finding in our study is the similar ultrafine particle generation between masked and unmasked tidal breathing (0.22 ± 0.29 and 0.15 ± 0.18 particles/mL, respectively, as measured with the PTrak). Asadi et al27 reported a 6-fold reduction in particle generation (0.3–20 µm) with masks compared to unmasked tidal breathing; however, subjects in that study performed expiratory maneuvers directly into a funnel that directed particles to the aerodynamic particle sizer. It would be expected that at least some proportion of particles would be diverted around the subject’s mask, missing the funnel and particle sizer. In our experimental setup, particles that were diverted around the subject’s mask may have been more likely to be counted. The discrepancy between masked and unmasked tidal breathing may also be related to random variation given the large confidence intervals or inherent measurement error in the particle counters, although similar findings were seen with both PTrak and Fluke counters.
There are several limitations to this study. First, most commercially available particle counters have an inherent measurement error for absolute particle concentration. Second, we were unable to assess particle composition and other biological properties that affect risk of aerosolization. Third, use of only healthy volunteers may limit the generalizability of the results. Lastly, small particle generation alone may not be a reliable surrogate to determine infectious risk. Future studies should include a larger sample size of subjects, including those with underlying pulmonary disease, to assess whether there are significant differences in aerosol generation in patients most likely to undergo peak flow and pulmonary function testing.
Conclusions
We developed an effective method to measure small particle generation after peak flow and pulmonary function testing. Mean particle concentrations increased with all devices used, but quantities were small compared to overall particle concentrations commonly found in the ambient environment. Our findings suggest that surgical masks and eye protection are likely sufficient infection control measures for office staff during peak expiratory flow testing in asymptomatic patients with well controlled respiratory symptoms. COVID-19 testing remains prudent in patients with acute respiratory symptoms prior to evaluation and peak expiratory flow assessment while the community prevalence of SARS-CoV-2 cases remains high.
Footnotes
- Correspondence: Alexander S Niven MD, Medical Co-Director, Pulmonary Function Laboratory, Division of Pulmonary, Critical Care, and Sleep Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905. E-mail: niven.alexander{at}mayo.edu
The authors have disclosed no conflicts of interest.
- Copyright © 2021 by Daedalus Enterprises