Abstract
Coronavirus disease 2019 (COVID-19) represents the greatest medical crisis encountered in the young history of critical care and respiratory care. During the early months of the pandemic, when little was known about the virus, the acute hypoxemic respiratory failure it caused did not appear to fit conveniently or consistently into our classification of ARDS. This not only re-ignited a half-century’s long simmering debate over taxonomy, but also fueled similar debates over how PEEP and lung-protective ventilation should be titrated, as well as the appropriate role of noninvasive ventilation in ARDS. COVID-19 ignited other debates on emerging concepts such as ARDS phenotypes and patient self-inflicted lung injury from vigorous spontaneous breathing. Over a year later, these early perplexities have receded into the background without having been reviewed or resolved. With a full year of evidence having been published, this narrative review systematically analyzes whether COVID-19–associated respiratory failure is essentially ARDS, with perhaps a somewhat different course of presentation. This includes a review of the severity of hypoxemia and derangements in pulmonary mechanics, PEEP requirements, recruitment potential, ability to achieve lung-protective ventilation goals, duration of mechanical ventilation, associated mortality, and response to noninvasive ventilation. This paper also reviews the concepts of ARDS phenotypes and patient self-inflicted lung injury as these are crucial to understanding the contentious debate over the nature and management of COVID-19.
- acute respiratory distress syndrome
- coronavirus disease 2019
- lung-protective ventilation
- noninvasive ventilation
- patient self-inflicted lung injury
Introduction
“Que sçais-je?” (“What do I know?”)
–Michel de Montaigne
With the exception of acquired immunodeficiency syndrome (AIDS), coronavirus disease 2019 (COVID-19) represents the greatest medical crisis the world has confronted since the “Great Influenza” pandemic of 1918. And certainly it is the most profound crisis in the young history of critical care and respiratory care. Even the AIDS epidemic did not remotely resemble the enormous strain on critical care capacity, health care provider staffing, and mechanical ventilators. However, this review of mechanical ventilation during the first year of the pandemic is not concerned with issues that captivated both mainstream and social media, such as the lack of ventilators. Rather its focus is the more interesting and deeper issue that animated the first months of pandemic and lingers still, perhaps forgotten or dismissed by many, but nonetheless one without definitive resolution or consensus.
At the pandemic’s onset there seemed to be a collective moment of self-doubt amid the terrifying chaos of COVID-19. Its apparently unusual presentation questioned how we apply the term ARDS and its ramifications on our approach to treatment. This uncertainty vaguely resembled controversies from the 1970s when the very idea of ARDS was considered by some “a distinctive non-entity” that “serves no useful purposes.”1 This is not to insinuate that in 2020 the validity of ARDS as an entity was being challenged, but rather the validity of what is encompassed by the definition. The specific characteristics of ARDS presentation have always engendered debate. The pandemic simply brought these long simmering issues to the forefront again. The basis for this was established in 2003 when the term “severe acute respiratory syndrome” (SARS) was coined rather than an alternative name in which ARDS was a salient feature.2 Naming has consequences.
Now with the perspective of time, the accruement of experience, data, and waning emotions, this narrative review is focused on our current understanding of COVID-19-associated respiratory failure and its response to mechanical ventilation. It also explores the controversies that arose in the early months of the pandemic. During this time frame, interesting opinions regarding both ARDS and COVID-19 were expressed, most based upon clinical impressions and interpretation of the scientific literature that deserve further exploration. These topics are consigned to supplementary materials for those interested (see the supplementary materials at http://www.rcjournal.com). For the primary topics of interest, the critique presented in this review focuses on how COVID-19 resembles or differs from our current understanding of ARDS. The intention is that we might answer the question the great Renaissance philosopher Michel de Montaigne posed to himself every day: What do I know?3
To Intubate or Not?
Two inter-related clinical management controversies arose almost immediately after the pandemic reached Europe and the United States. The first was whether patients with respiratory insufficiency should be intubated before exhibiting signs of overt failure.4,5 The second was whether an apparently unusual presentation of COVID-19 respiratory failure was indeed ARDS, thereby raising questions whether the approach to invasive ventilation should be modified in response.6-8 These controversies influenced how respiratory care was practiced over the first year of the pandemic.
The rationale for early invasive ventilation was based upon 3 factors. First, fear regarding potential aerosolization due to managing patients either with noninvasive invasive ventilation (NIV) or high-flow nasal oxygen.9-11 Clinicians involved with aerosol-generating procedures have ∼ 3 times the infection risk compared to other health care professionals.12 Early on, the infection rate among health care workers was ∼ 4% in China (the majority in Wuhan) and 14% in Italy.13,14 Second was a concern for the potential development of patient self-inflicted lung injury caused by spontaneous breathing at a supranormal tidal volume (VT) generated by high transalveolar pressures (> –15 cm H2O) from a combination of high respiratory drive, preserved respiratory muscle strength, and near-normal lung volumes.7 Hypothetically, early intubation and control of the ventilatory pattern might mitigate the severity of respiratory failure.15,16 Third, early reports from China described sudden, acute respiratory destabilization in 46–65% of patients with COVID-19 in the ICU,17,18 raising apprehension of delayed detection in overwhelmed hospitals.15,19,20 Thus preemptive intubation appeared reasonable from a safety perspective.
The counterargument, colloquially referred to as “avoid intubation at all costs,”21 was largely driven by the following rationale. Early on invasive ventilation was associated with extraordinarily high mortality (70–100%).22-25 Also, severely hypoxemic patients initially appeared stable, with relatively intact pulmonary mechanics and respiratory muscle reserve, often without apparent respiratory distress (“silent hypoxemia”).5,26 Again, in the context of overwhelmed clinicians and a looming (sometimes actual) shortage of ventilators, forestalling intubation with noninvasive respiratory therapies appeared rational and pragmatic.8 In terms of infection control, the evidence, as it existed, strongly suggested that the primary risk for clinician infection was not NIV or high-flow nasal oxygen, but rather intubation and associated periods of bag-mask ventilation.27
Is This Really ARDS?
“Taxonomy is described sometimes as a science, sometimes as an art, but really it’s a battleground.”
–Bill Bryson28
The second controversy was that COVID-19-induced respiratory failure differed substantially from ARDS. This raised questions whether invasive ventilation practices should deviate from current evidence-based lung-protective ventilation (LPV) guidelines and protocols. The controversy ranged from circumspect, well-reasoned, tentative opinions (based upon decades of ARDS research)7,8 to skewed interpretations regarding the Berlin definition criteria for syndrome onset,29 to ill-informed conjecture such as COVID-19 resembling high-altitude (ie, “hydrostatic”) pulmonary edema rather than altered permeability pulmonary edema (the quintessential feature of ARDS).30
Whether COVID-19 respiratory failure differs from ARDS should, as a first step, refer back to the definitions of taxonomy and syndrome. Taxonomy refers to how phenomena are organized or classified according to common attributes. By its nature, taxonomy is rule-based, which to some degree is unavoidably arbitrary and thus prone to controversy. Syndrome, derived from the Greek word for “concurrence,” refers to a set of co-related signs and symptoms associated with a particular disease or disorder. ARDS represents an effect emanating from a multitude of potential initiating sources causing acute pulmonary tissue injury and an inflammatory response. These result in varying degrees of severity of both epithelial and endothelial injury, altered permeability pulmonary edema, altered lung mechanics, and hypoxemia.
As such, the definition of ARDS requires that it be based on common attributes for making a classification when numerous pathogenic agents can initiate lung injury, these attributes being: (1) a specific threshold of oxygenation dysfunction using the ratio of arterial partial pressure of oxygen to inspired oxygen fraction (PaO2/FIO2) ≤ 300 mm Hg (ie, an approximation of the traditional hypoxemia threshold of ∼ 60 mm Hg on room air); (2) radiographic presentation of bilateral lung opacities suggestive of disseminated alveolar injury; and (3) an inciting mechanism (etiology) known or suspected to cause acute lung injury.
Although the definition of ARDS has evolved since 1967 (albeit with controversy), these defining characteristics have not fundamentally changed. Most relevant to COVID-19 is that viral pneumonia accounted for 33% of subjects first described as having ARDS in the seminal 1967 paper by Ashbaugh et al.31 In addition, evidence suggests that ARDS was the primary cause of early mortality during the 1918 H1N1 pandemic.32 Since 1967, multiple viruses have been associated with the syndrome, including influenza, adenovirus, varicella, hantavirus, and coronavirus.2 In early reports from China, 65–85% of patients with COVID-19 who were admitted to the ICU met ARDS criteria.33,34
Part of the controversy rests with the fact that radiographic evidence of ARDS has always been the most vulnerable criterion given the high degree of inter-observer variability (even among experts).35 In addition, a telling observation was that radiologically “COVID-19 lung involvement is unique having a pneumonia pattern rather than a typical ARDS pattern at least in the initial phase during the first days after intubation” [italics added].36 Implicit in this statement is that severe hypoxemia was associated with initial lobar pneumonia. In addition, the speed of acute lung injury progression in viral ARDS is dependent upon the speed of viral replication, which differs between viruses (eg, H1N1 vs SARS CoV-1),32 and perhaps between SARS CoV-2 variants as well. Another underlying contributing factor has been the tendency toward under-recognition of ARDS in clinical practice.37
Finally, a misreading of Berlin definition criteria likely played a role. A review paper cited 3 early studies from China in which the median time from symptom onset to ARDS was 8–12 d.29 Although the time frame exceeds the criterion established by the Berlin Definition Taskforce,38 the authors did not use the full description, which included “or new or worsening respiratory symptoms” (ie, underlying disease progression as alluded to above). Interestingly, the “7 d from onset” criterion was based on a single-center study of 182 subjects with risk factors who subsequently developed ARDS, but excluded pneumonia as a risk factor.39 Between 35% and 56% of subjects enrolled into large prospective ARDS treatment trials had pneumonia as the primary etiology, thus limiting the external validity upon which the 7-d criterion was initially based.40-44
The Theory of ARDS Phenotypes
Phenotypes are the observable characteristics of an organism (eg, physical, morphologic, biochemical), whereas genotype refers to an organism’s entire catalogue of genes available for potential expression. Phenotypes represent an interaction between the organism’s genotype and the environment it encounters. Specific to ARDS, this would include infectious or other injurious agents and the therapies used to treat it (eg, invasive ventilation, hyperoxia, pharmacologic agents). In COVID-19-associated ARDS, use of the term phenotype created more controversy than clarity.45-50 Regardless of etiology, individual responses to acute lung injury exist along a spectrum, ranging from mild to severe, that involves the interplay of several factors.
In ARDS, phenotypic expression would encompass either the propensity or disinclination for developing a hyperimmune response to acute lung injury (ie, cytokine storm syndrome).51,52 An individual’s genetic susceptibility would also apply to the propensity for developing hyperoxic acute lung injury53 and ventilator-induced lung injury.54 Prior to COVID-19, interest in ARDS phenotypes focused on apparent hypo- or hyperinflammatory (ie, reactive) responses to acute lung injury. Hyperinflammatory phenotypes are thought to occur in ∼ 33% of ARDS cases, are associated with severe ARDS, and perhaps are more responsive to PEEP, certain pharmacologic therapies, and conservative fluid management.55-57
However, it is difficult to disentangle an individual’s response to COVID-19-induced lung injury from numerous inter-related factors such as the magnitude of infectious insult (including the potential impact of SARS CoV-2 variants), the usual stages of pneumonia progression,50 the presence of comorbidities, abnormal body habitus (ie, the extent to which it exaggerates hydrostatic forces that worsen chest mechanics, gas exchange and radiographic findings), and the intensity and duration of exposures to hyperoxia and injurious ventilation patterns. There also exists the inherent problems of conducting physiologic research in the critical care setting (eg, selection bias, small sample sizes), problems that are magnified under pandemic conditions.
The most succinct criticism of phenotyping COVID-19 was that it was premature.46 First and foremost, it preceded systematic, unbiased data collection that ultimately leads to “a phenotypic signature specific to high gene expression.”46 Second, the attempt was based on single-center data and “anchored on only one or two clinically apparent variables.”46
COVID-19 Phenotypes
The COVID-19 phenotypes hypothesis was raised early on in editorials on the basis of observations initially made in an undisclosed number of subjects, and subsequently reported as being made in 150 subjects.7,8 The basis was severe hypoxemia dissociated from corresponding reductions in respiratory system compliance (CRS) usually observed in ARDS. Consequently it was proposed that COVID-19-associated respiratory failure be classified as non-ARDS (Type 1) and ARDS (Type 2).8 Of note, the term “non-ARDS” was quickly modified to “atypical ARDS.”58
In Type 1 COVID-19, computed tomography (CT) imaging showed essentially normal gas volume and minimal (∼ 8%) non-aerated lung tissue associated with normal CRS (80 mL/cm H2O) and disproportionately elevated venous admixture (56%). This was attributed to severe ventilation-perfusion mismatching caused by loss of compensatory hypoxemic vasoconstriction (from viral injury of the pulmonary vascular endothelium), rather than intrapulmonary shunt from large amounts of nonaerated tisssue.7 In contrast, Type 2 COVID-19 exhibited a classic ARDS profile with markedly reduced lung volume (∼ 60% of normal) with 39% nonaerated lung tissue and both venous admixture and CRS typically found in ARDS (49% and 43 mL/cm H2O, respectively).
The proposed phenotypes were later renamed from Type 1 to Type L (ie, low lung elastance or high “preserved” lung compliance) and from Type 2 to Type H (ie, high lung elastance or low lung compliance) on the basis of data culled from 150 subjects.7 In addition to describing these archetypal presentations of COVID-19 respiratory failure, the authors (as well as others) suggested a modified approach to ventilator management (Table 1).7,15,20
Proposed COVID-19 Phenotypes of Respiratory Failure and Early Management Recommendations*
COVID-19 Phenotypes and LPV
The ensuing controversy over modifying LPV for COVID-19 focused primarily on liberalizing VT in steps from 6 mL/kg to 7, 8, and perhaps 9 mL/kg when hypercapnia or severe dyspnea were present, and only in those patients presenting as Type L.7,8 In other words, this approach would be applied to patients in whom lung volume is well preserved so that the risk of developing ventilator-induced lung injury (VILI) would be relatively minor and a reasonable trade-off to balance other risk factors.
Liberalized VT within accepted LPV parameters has been a consistent feature of European studies for decades.59-67 In addition, the 2016 LUNG SAFE international survey also used 8 mL/kg as the upper threshold for LPV.63 Moreover, the Surviving Sepsis Campaign Guidelines for COVID-19 recommended a VT of 4–8 mL/kg.68 The insinuation that these circumscribed guidelines deviated from accepted LPV norms was highly misleading.46,69 Furthermore, these recommendations are in stark contrast to others who suggested COVID-19 can be managed safely with a VT ≤ 11 mL/kg (assuming that plateau pressure was ≤ 32 cm H2O).6,70
Reasonable liberalizing of VT from 6 to 7–8 mL/kg was based upon observations that it often attenuates dyspnea8 and is supported indirectly by studies on VT demand during LPV (see the supplementary materials at http://www.rcjournal.com).71 A peculiar aspect of arguments against liberalizing VT72,73 is that they conveniently ignored discussing the reliance upon sedation to control dyspnea and asynchrony, which also carries substantial risk of harm.74-76 A decade ago, evidence suggested that patient-ventilator asynchrony was associated with worse outcomes,77 and more recent evidence suggests that persistent, severe patient-ventilator asynchrony may be particularly harmful.78 In this context, the issue of whether patient self-inflicted lung injury is a factor in COVID-19 progression (and its potential exacerbation by dyspnea frequently associated with VT-mismatching during LPV) raises legitimate cause for concern.
The second controversy focused on how PEEP should be applied. The Surviving Sepsis Guidelines for COVID-19 suggesting a higher PEEP strategy over a lower PEEP strategy (ie, PEEP > 10 cm H2O) drew particular criticism.68 In response, an editorial pointing out the vague nature of the criticism replied that “higher PEEP does not necessarily imply very high levels of PEEP.”79 That statement was made in the context of remarking upon a small PEEP study for which it was written.80 In that study, borderline super-PEEP (18 cm H2O) applied in Type L subjects with relatively preserved CRS (58 mL/cm H2O) markedly improved oxygenation and end-expiratory lung volume but at the predictable expense of overdistention and hemodynamic impairment.80 Similarly, investigators in Greece observed relatively preserved CRS (50–65 mL/cm H2O) with median “best PEEP” levels of only 8 cm H2O. This led them and others to criticize use of pre-defined PEEP such as the ARDSNet PEEP/FIO2 tables and recommended their abandonment in most COVID cases.6,36,81
Phenotypes Versus Disease Evolution in COVID-19
Early reports regarding COVID-19 phenotypes were limited by the lack of specific data despite claims that this idea was based on “detailed observation of several patients and discussions with colleagues” and “more than 50% of the 150 patients measured by the authors and confirmed by several colleagues in Northern Italy.”7 This initial description was quickly followed by specific data from 16 subjects showing that mean CRS of 50 ± 14 coincided with mean pulmonary shunt of 0.50 ± 0.11.58 Yet the first detailed mechanical ventilation study from Italy on COVID-19 phenotypes did not appear until October 2020 and included data from only 32 subjects.67
A striking comment was that COVID-19-associated ARDS “as the same disease” presents itself with impressive non-uniformity and that such a wide discrepancy (between magnitude of hypoxemia and corresponding severity in reduced CRS) is almost never seen in severe ARDS.7,58 These observations were accompanied by pro forma statements listing potential confounding factors such as the combined effects of infection severity and host response, variability in individual responses to hypoxemia, and (particularly crucial to their hypothesis), that the duration between disease onset and observation would lead to a time-related disease spectrum with 2 primary “phenotypes.”7
In other words, COVID-19 ARDS likely evolves over time and transitions from a mild to a severe phenotype that, based on the timing of presentation (scientific observation), may present “insurmountable methodological challenges” to study.7,82 But liberalizing the definition of ARDS phenotypes from hypo- versus hyperimmune response to one suggesting that apparent variations in COVID-19 expression somehow fundamentally differ from the non-uniformity observed in ARDS (irrespective of etiology) is highly suspect in its reasoning (see the supplementary materials at http://www.rcjournal.com).
Conflicting Evidence Regarding COVID-19 Phenotypes
Last September, data from 38 subjects with COVID-19-associated ARDS contradicted the idea of phenotypes.83 In these subjects, chest CT imaging (using nonquantitative analysis) performed directly after intubation revealed that only ∼ 35% met either Type L or Type H criteria. The majority represented discordant results regarding the lack of association between CRS and the amount of poorly or nonaerated tissue, suggesting wide overlap in presentations.
The following month, proponents of the COVID-19 phenotype concept published an in-depth study on the gas exchange, pulmonary mechanics, and CT findings alluded to in their early editorials.67 In this case-control comparison, subjects with confirmed COVID-19-associated ARDS were matched 1:1 with 2 separate non-COVID-19 ARDS cohorts by PaO2 and by CRS. CT quantitative analysis of lung tissue was performed at a standardized PEEP of 5 cm H2O (ie, removing the confounding effects of therapeutic lung recruitment from assessing baseline pathophysiology). Subjects with ARDS due to COVID-19 shared similar amounts of poorly aerated lung tissue with subjects in the PaO2/FIO2 -matched ARDS cohort, but in almost every other aspect they more closely resembled subjects in the CRS-matched ARDS cohort (see the supplementary materials at http://www.rcjournal.com).
The discrepancies between these studies reflects the inevitable limitations imposed by small sample sizes. Possible differences between the studies likely included timing of measurements relative to disease onset. This is particularly relevant given radiographic reports that rapid progression of lesions was sometimes observed.84,85 The lack of standardization of ventilator settings in 1 trial83 and differences between non-quantitative and quantitative analysis of CT scans across the studies may have influenced their interpretation.
Pathologic and Radiologic Features of COVID-19
Finally, the existence of proposed COVID-19 phenotypes is inextricably tied to the declaration that they represent a time-related disease spectrum.7 Such a statement requires reviewing both the pathologic and radiologic evidence in COVID-19-associated respiratory failure. A brief letter describing 6 postmortem exams observed that COVID-19-associated lung injury progressed over time.86 Findings in subjects who died 5 d after symptom onset revealed lymphocytic pneumonia with both interstitial and alveolar infiltration consistent with a Type L presentation. The 5 other subjects who died at ∼ 20 d all presented with acute fibrinous organizing pneumonia and extensive intra-alveolar and bronchiolar involvement, as well as endothelial injury consistent with Type H presentation.
A subsequent study of 41 subjects compared histopathologic findings between subjects who died at varying time points.87 Findings among subjects who died within the first 8 d differed from those who died afterwards. The first cohort exhibited a predominantly exudative pattern with interstitial and intra-alveolar edema and varying degrees of alveolar hemorrhage, fibroblastic proliferation, and hyaline membrane formation. Subjects who died 17–40 d after symptom onset largely presented with fibroblastic proliferation with densely fibrotic areas. Across study time frames, pulmonary microthrombosis was frequently observed. The histopathologic pattern and time-dependent evolution of diffuse alveolar damage found in subjects with COVID-19-associated ARDS was “stereotypical” of that observed in non-COVID-19-associated ARDS.87 Another study observed an early stage characterized by neutrophilic, exudative capillaritis with microthrombosis in contrast to a later stage with a classic ARDS presentation of diffuse alveolar damage and ongoing intravascular thrombosis in small to medium sized vessels.88
Radiologic findings regarding COVID-19 progression were consistent with those found at autopsy. CT imaging in 63 subjects was compared between initial examination and reexamination between days 3–14.85 Initial examination noted that 30% of subjects had only single lobe involvement, whereas ∼ 55% had involvement in 4–5 lobes with patchy/punctate ground glass opacities as the primary characteristic. Reexamination revealed variable (sometimes rapid) disease progression with diffuse lesions of increasingly dense ground glass opacities and tissue consolidation (ie, “white lung”). The investigators’ general impression was that CT imaging of COVID-19 was similar to common viral pneumonia.85
Renin-Angiotensin System and Hypoxemia in COVID-19
Dysregulation of compensatory hypoxemic pulmonary vasoconstriction in Type L phenotype aligns with the fact that SARS CoV-2 pulmonary infection primarily targets angiotensin-converting enzyme 2 receptors (ACE II) of the pulmonary endothelium.89 In brief, ACE II receptors are part of the renin-angiotensin system in which the hormone angiotensin causes vasoconstriction. ACE is abundantly produced by the capillary endothelium and plays a major role in maintaining ventilation-perfusion balance in response to hypoxemia.90 ACE II receptors also are found in both airway and alveolar epithelial cells, with emerging evidence that angiotensin plays a prominent (albeit complicated) role in the inflammatory response to both ARDS and ventilator-induced lung injury.90
An alternative explanation is that infected alveolar epithelial cells downregulate ACE II activity causing unopposed ACE I activity in neighboring endothelial cells. Although this would trigger a disproportionate release of endothelin-1 (a potent pulmonary vasoconstrictor) causing recruitment of pulmonary capillary beds,50 the end result would be similar: severe hypoxemia from ventilation-perfusion mismatching.
Observation and Interpretation During a Global Medical Crisis
Thus, both pathologic and radiographic findings suggest that what was initially interpreted as different COVID-19 phenotypes appears simply to be disease progression. This is likely attributable to a confluence of factors, including the timing of observation relative to a variable disease progression. More importantly, scientific inquiry normally affords the luxury of open-ended contemplation prior to publication. The COVID-19 pandemic afforded no such luxury. Enormous pressure likely was felt by preeminent ARDS researchers to quickly make some sense of their preliminary observations and convey them to a global audience struggling to understand, let alone manage, a novel viral pandemic. These observations appear concordant with those penned by Dr Gattinoni and colleagues toward the end of 2020.91
The Theory of Patient Self-Inflicted Lung Injury
The earliest description of COVID-19 ARDS pathogenesis posited that a minority of patients (20–30%) who either initially presented as (or later transitioned to) Type H phenotypes may have had their disease course exacerbated by patient self-inflicted lung injury from spontaneous breathing at a supranormal VT and high transalveolar pressures.7 Prolonged inspiratory efforts resulting in both excessive pleural pressure swings ≥ 15 cm H2O and VT (≥ 15 mL/kg) were proposed to cause or perpetuate acute lung injury.7 Because severe SARS CoV-2 infection involves the vascular endothelium, it was further suggested that the carotid bodies may become hypersensitive to hypoxemia, causing abnormally heightened respiratory drive (ie, disproportionate to the severity of hypoxemia) and thus contributing to patient self-inflicted lung injury.92
Strenuous diaphragmatic contractions would normally cause high negative pleural pressures to be transmitted homogeneously across healthy lungs (ie, fluid behavior), thus minimizing abnormal strain-stress development. But heterogeneously injured lungs dissipate pressure un-evenly, so that stress becomes amplified at the interfaces between collapsed/consolidated tissue and surrounding normally aerated tissue (ie, solid behavior). This results in greater inflammation and edema formation, particularly in dependent lung regions.93
Preclinical evidence has demonstrated that high VT ventilation generated by negative transpulmonary pressure induces acute lung injury in normal lungs.94,95 In acutely injured lungs undergoing assisted ventilation, doxapram-induced inspiratory efforts resulting in only a moderate VT (∼ 8 mL/kg) but transpulmonary pressures ≥ 30 cm H2O produced the greatest degree of lung collapse, hyperinflation, and histologic injury within a matter of only 4 h.96
Clinical evidence supporting patient self-inflicted lung injury remains speculative. First, in both COVID-19-associated and non-COVID-19-associated ARDS alike, patient self-inflicted lung injury would likely follow the 2-hit theory of lung injury, whereby the initial insult would prime the immune system, with subsequent high stress-strain ventilation further intensifying inflammation.97,98 Second, a relatively safe plateau pressure (Pplat) of ≤ 30 cm H2O, traditionally advocated for LPV, assumes normal chest wall compliance, so that the projected peak transalveolar stress would not exceed 20 cm H2O.99 In addition, tidal stress change (ie, Pplat – PEEP > 15 cm H2O) has been shown to increase mortality risk.100 But when examining Figure 2 from the study by Amato et al,100 it is apparent that the inflection point for mortality risk becomes pronounced only at ∼ 20 cm H2O, which was associated with a median VT of 8 mL/kg.
Finally, the plausibility of patient self-inflicted lung injury has been documented in acute lung injury. Spontaneous breathing efforts during assisted ventilation in pneumonia or non-pulmonary sepsis produced median (interquartile range [IQR]) transpulmonary pressures of 18 (IQR 14–23) cm H2O.101 Likewise, median (IQR) negative esophageal pressure swings of 17 (IQR 12–22) cm H2O have been reported during unassisted breathing in ARDS, with individual measurements as high as 31 cm H2O.102 Subjects recovering from COVID-19-associated ARDS generated large negative intrathoracic pressures during weaning.103 Of particular interest, subjects who developed relapse respiratory failure 24 h after a weaning trial generated greater negative pressure swings than those who did not (18 [IQR 15–26] vs 15 [IQR 7–18] cm H2O), and several subjects generated pressure swings ≥ 30 cm H2O.103 In subjects with acute hypoxemic respiratory failure (78% with ARDS), generating a spontaneous VT > 9.5 mL/kg was independently associated with NIV failure.104 Moreover, it was observed that maintaining a VT of 6–8 mL/kg was possible in only 23% of subjects despite pressure support levels used in spontaneous breathing trials (ie, 7 cm H2O). This underscores the general difficulty of maintaining LPV goals in critically ill patients with heightened respiratory drive.
Invasive Ventilation Usage and Associated Mortality
Concern during the first months of the pandemic focused on extraordinarily high mortality associated with invasive ventilation. This was based largely on 4 studies with < 500 cases.22-25 That Chen and colleagues25 reported all 17 invasively ventilated subjects died may have garnered disproportionate attention.
By the end of 2020, a large number of studies that included data on invasive ventilation had been published (see the supplementary materials at http://www.rcjournal.com).22-25,34,105-127 Regarding the need for invasive ventilation, 32 observational studies with > 15,000 subjects reported median (IQR) usage of 23% (IQR 13–54%) with a corresponding mortality of 49% (IQR 31–70%). Some of the highest mortality rates (≥ 80%) were reported early on from countries and regions ravaged by the pandemic.24,25,34,105,126 These represented the least prepared areas and prior to discovering effective pharmacologic therapies.128
Because it was imperative to disseminate even preliminary information during the crisis, more than half of these studies ceased data collection prior to hospital discharge and before establishing definitive outcome data. An international meta-analysis attempted to compensate for this by estimating both the lowest and highest possible mortality rates (ie, assuming all outstanding cases either survived or succumbed to COVID-19).129 These estimates ranged from a lowest mortality rate of 43% (95% CI 36–51%) to a highest mortality rate of 64% (95% CI 56–72%). When restricted to completed outcome data, the mortality rate was 49.5%. Another international study focused on hospital mortality differences on the basis of “organ support.”130 Among hospitalized subjects who did not require invasive ventilation, renal replacement therapy, or vasopressor therapy, the mortality rate was only 8%. In contrast, the mortality rate was 40.8% in those who required only mechanical ventilation and increased to 71.6% in those who required dialysis and vasopressor support (ie, multiple organ dysfunction syndrome).
For perspective, observational studies of ARDS in the LPV era have reported 95% CI for mortality of 31–39% (mild), 37–43% (moderate), and 42–50% (severe).63 Similar to COVID-19, when ARDS was associated with renal failure, mortality risk increased to 80% in some studies.131 COVID-19 mortality associated with invasive ventilation is similar to that observed during the SARS CoV-1 pandemic (45–48%),132,133 and lower than that observed with the Middle East respiratory syndrome coronavirus (MERS-CoV) epidemic (60–74%).134-136
Invasive Ventilation Duration
Prolonged invasive ventilation has also been observed with COVID-19.110 In the aforementioned studies, 16 reported duration as it pertained to survivors, time to first successful extubation trial, or the presence of multiple-organ system dysfunction. With one exception, central tendency exceeded a week.117 Another study reported that duration was not appreciably different between survivors and nonsurvivors; moreover, in subjects intubated following NIV failure, mean duration increased from 15 to 17 days.125
Acute kidney injury and the need for renal replacement therapy had a variable impact on invasive ventilation duration depending upon outcome.137 Acute kidney injury alone increased median duration for all subjects versus survivors by 2.5 and 3.5 d, respectively. Among those also requiring dialysis, overall median duration was unaltered (14 d) but increased substantially between survivors who required dialysis therapy compared to survivors who did not require dialysis: 28.6 (IQR 21.1–37.2) versus 15.0 (9.1–19.6) d.
This exemplifies the problem with collecting data during a pandemic. The urgent need for information virtually compels reporting incomplete outcome data distinct from established norms (eg, status at hospital discharge or at day 90). As a consequence, the interpretation of invasive ventilation duration (or associated mortality) can be misleading. In one study, 35% of subjects successfully extubated had a median intubation duration of 10 (IQR 6–15) d, whereas 65% remained ventilator-dependent with median intubation duration of 18 (IQR 14–24) d when data collection stopped.110
For perspective, in randomized controlled trials of LPV in ARDS (wherein comorbidities are largely removed as a factor), the mean or median duration of invasive ventilation for lower versus higher PEEP strategies was similar to those reported for COVID-19, respectively: 13.5 and 14.2 d,40 21 and 25 d,44 10 d each,43 and 22 and 17 d.138 In addition, a large observational study of weaning subjects with ARDS either by spontaneous breathing trials with daily sedation interruptions or usual care practices produced median findings within the range reported in COVID-19: 9 (IQR 4–17) and 14 (IQR 6–29) d, respectively.139
PEEP and VT Parameters
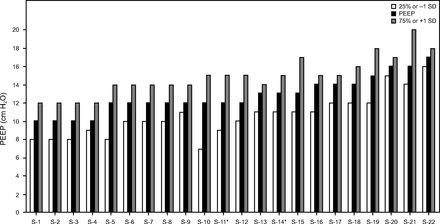
Twenty-three reviewed studies provided initial ventilator data (Table 2).83,106,107,110,113,115,116,120,127,140⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-153 In 22 of these, mean/median PEEP requirements were 10–16 cm H2O (Table 2, Figure 1). A crude approach for determining the need for particularly high PEEP levels (ie, approaching the “super-PEEP” threshold of 20 cm H2O) are values demarcating 1 SD above the mean, or the 75th percentile. In only 4 (18%) studies did these demarcation thresholds exceed 16 cm H2O, and only one reached 20 cm H2O.127,143,147,149 By comparison, lower range PEEP requirements (ie, demarcated by 1 SD below the mean or 25th percentile) were twice as frequent with 36% of studies reporting values < 10 cm H2O. For perspective, general PEEP requirements in ARDS during LPV are 10–18 cm H2O for the vast majority of patients.154 These findings suggest that PEEP requirements in COVID-19-associated ARDS are not different from the general ARDS population.
Distribution of baseline PEEP requirements during invasive ventilation ordered from lowest to highest mean or median values (S denotes only the study order). Dispersion of values as either 1 SD above/below the mean or the 25th/75th percentile.
Mechanical Ventilation Characteristics
Among 18 reviewed studies reporting VT in mL/kg, 94% found mean/median values < 8 mL/kg, with 78% with values < 7 mL/kg (Table 2, Figure 2). Using the demarcation points described above, violation of LPV VT parameters (ie, > 8 mL/kg) was reported in only 17% of studies,67,110 which suggests that VT management in COVID-19 was largely achieved within accepted LPV guidelines and liberalization was not widely practiced.
Distribution of baseline tidal volume (VT) during invasive ventilation ordered from lowest to highest mean or median values (S denotes only the study order). Dispersion of values as either 1 SD above/below the mean or the 25th/75th percentile.
Respiratory System Compliance
Type L COVID-19 (ie, atypical ARDS) was observed in 70–80% of ventilated subjects in Italy during the first months of the pandemic. The salient characteristic were relatively preserved CRS (ie, > 50 mL/cm H2O) versus Type H (ie, typical ARDS) demarcated by CRS < 40 cm H2O observed in only 20–30% of subjects.8,58 Given that context, studies with timeline data accompanying invasive ventilation characteristics reported that intubation occurred from 0–7 d after hospital admission, with baseline observations proceeding soon afterwards (ie, mostly subjects with early ARDS).107,115,120,141,146,152,153,155
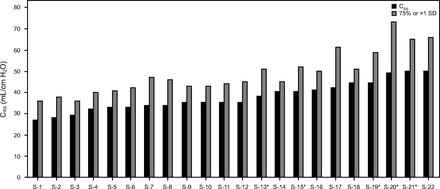
In 68% of reviewed studies, the central tendency for CRS was ≤ 40 mL/cm H2O, and in only 9% did it reach 50 cm H2O.67,69,106,107,110,113,115,120,127,141,143-152,156,157 This finding was similar to that for non-COVID-19-associated ARDS managed with LPV (32–38 mL/cm H2O)40,44,158-160 but higher than that for ARDS studies preceding LPV (30–34 mL/cm H2O).161 CRS values at 1 SD above the mean or the 75th percentile ≥ 50 mL/cm H2O were reported in 43% of studies (Figure 3).67,69,106,144,146,147,152 However, with one exception,67 the corresponding PEEP levels were 12–20 cm H2O; thus the relevance of higher CRS in assessing Type L prevalence remains uncertain. In the largest study focused on COVID-19 lung mechanics, CRS decreased over 14 d from 38 ± 11 to 31 ± 14 mL/cm H2O.150 This was consistent with COVID-19 pathology examination patterns in which diffuse exudative patterns were prominent early on (ie, hospitalization day 0–8) and were replaced by pronounced fibroproliferative patterns afterwards.87
Distribution of baseline respiratory system compliance (CRS) during invasive ventilation ordered from lowest to highest mean or median values (S denotes only the study order). Dispersion of values as either 1 SD above the mean or the 75th percentile.
Thus, contrary to initial reports from Italy, CRS was not well preserved. Even the higher dispersion of CRS values mostly corresponded to higher PEEP (14–20 cm H2O), which likely improved CRS relative to what was measured prior to PEEP titration (eg, conventional initial PEEP of 5 cm H2O).67 Nonetheless, the puzzling observations of preserved CRS reported in Italy were also reported anecdotally in nearby Greece.36,81 This raises an interesting possibility that perhaps a since-displaced CoV-2 variant circulating early on in Southern Europe might have had relatively slower replication, and thus slower progression of lung injury.
Lung and Chest Wall Compliance
Prior to the advent of LPV, pathologic alterations in lung and chest wall compliance were measured in numerous studies. In studies reporting mean CRS of 30–34 mL/cm H2O, corresponding mean lung and chest wall compliances were 32–72 mL/cm H2O and 59–147 mL/cm H2O, respectively, representing reductions of 40–60% and 50–80% from normal, respectively.161
Only 2 studies have reported lung and chest wall compliance in COVID-19. In a study in which median PEEP was 14 (IQR 12–15) cm H2O, corresponding median values for CRS, lung compliance, and chest wall compliance on the first day of invasive ventilation were 32, 41, and 154 mL/cm H2O, respectively; these values were consistent with historical values reported in ARDS.152 The other study collected data within 48 h of intubation at a median PEEP of 10 (IQR 8–12) cm H2O.146 Although the median CRS (44 mL/cm H2O) was higher than historical values, both median lung compliance and chest wall compliance (59 and 144 mL/cm H2O, respectively) were consistent with corresponding historical values. On the basis of these limited data, pathologic alterations in both lung compliance and chest wall compliance in COVID-19 were similar to those reported in non-COVID-19-associated ARDS.
Interplay of Oxygenation, PEEP, and Compliance
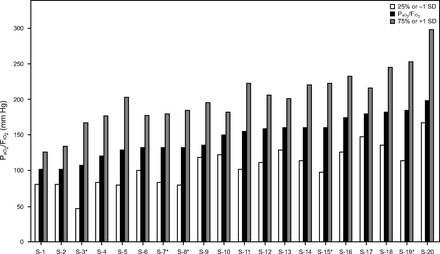
In the early phase of COVID-19–associated ARDS, oxygenation fell within the Berlin definition boundaries of moderate ARDS with PaO2/FIO2 central tendencies across most studies of 101–198 mm Hg.67,69,106,107,110,113,115,116,120,127,140,141,144-148-150,152,153,157 Using the previously described lower and upper demarcation criteria, 40% of studies had PaO2/FIO2 ≤ 100 mm Hg, whereas 55% had PaO2/FIO2 > 200 mm Hg (Figure 4).
Distribution of baseline (arterial oxygen tension-to inspired oxygen fraction) during invasive ventilation ordered from lowest to highest mean or median values (S denotes only the study order). Dispersion of values as either 1 SD above/below the mean or the 25th/75th percentile.
The relevance of these data obviously is limited by the corresponding PEEP at these demarcated boundaries. For 16 studies that also reported PEEP data, there were 6 studies in which lower PaO2/FIO2 boundaries represented severe ARDS, and the corresponding PEEP boundaries were 7–11 cm H2O; in 5 of these studies PEEP boundaries were < 10 cm H2O.69,107,110,146,148,150 In 9 studies reporting upper PaO2/FIO2 boundaries representing mild ARDS, the corresponding PEEP boundaries were 12–18 cm H2O; in 8 of these studies these boundaries were ≥ 14 cm H2O.106,113,115,116,122,127,141,144,149 The relationship between central tendencies of PaO2/FIO2and PEEP across these studies showed a moderately high correlation (r = 0.77 [95% CI 0.56–0.88], P < .001). This suggests initial oxygenation defects reported in COVID-19 mostly reflected how PEEP was being used rather than providing an accurate assessment of the underlying oxygenation defect. Moreover, it appears that PEEP levels required to stabilize oxygenation in COVID-19-associated ARDS are not different from those used in non-COVID-19-associated ARDS.
Lung Recruitment Potential
Lung recruitment potential in ARDS is multifactorial, with both limited application and variable efficacy. Efficacy depends more upon both the timing of recruitment relative to ARDS evolution (ie, early exudative vs later fibroproliferative phase) and the severity and distribution of lung injury (ie, diffuse vs lobar patterns) than on lung injury etiology.162 Five studies assessed recruitment potential in COVID-19-associated ARDS using a 10 cm H2O increment or decrement in PEEP (see the supplementary materials at http://www.rcjournal.com).80,146,153,163,164
Four studies used the recruitment-to-inflation ratio (R/I) to assess recruitment potential. Briefly, immediately following the sudden application or withdrawal of PEEP, expired VT will decrease or increase, respectively, compared to prior breaths. This is because gas is either trapped by increased PEEP or released by decreased PEEP. The trapped or released volume represents changes in end-expiratory lung volume, so that recruitment compliance is calculated as expired ΔV/ΔPEEP. This value is compared to CRS measured at a PEEP of 5 cm H2O (ie, compliance of the baby lung) on the basis of the assumption of linear CRS without changes in aerated lung units.165 The R/I validation study indicated that values ≥ 0.5 were indicative of high recruitment potential, whereas values < 0.5 indicated poor recruitment potential.165
Four studies assessing R/I in COVID-19 presented evenly divided results, each reporting either poor or good recruitment potential. Yet most studies noted a wide range of individual R/I values.146,153,164 Those with the lowest recruitment potential were studied in the fibroproliferative stage of ARDS and had extremely low mean CRS (20 cm H2O).163 Similarly, Beloncle et al153 reported that when R/I was repeated 5 d later, 30% of those initially classified as having high recruitment potential had transitioned to low recruitment potential with a corresponding decline in CRS.
Two of 5 studies that recorded CRS at each PEEP level observed that oxygenation and end-expiratory lung volume increased markedly at higher PEEP levels despite exhibiting both declining CRS and elevated stress index.80,164 This suggested recruitment occurred simultaneously with regional overdistention. Overall, the findings of recruitment potential in COVID-19-associated ARDS are consistent with those in non-COVID-19-associated ARDS, specifically the timing of recruitment relative to ARDS onset.162
Role of NIV in ARDS and Viral-Induced ARDS
Managing ARDS with NIV is controversial as the syndrome itself independently predicts therapeutic failure,166 with overall intubation rates of 30–61% in some studies.104,166-172 In other studies, NIV failure rose with increasing ARDS severity from 19–22% (mild), 42–73% (moderate), and 47–84% (severe).166,169,170 In addition, specific PaO2/FIO2 nodal points of < 150 mm Hg,104,166,167,170,172 < 175 mm Hg,168 and ≤ 179 mm Hg169 are associated with NIV failure. NIV failure is strongly associated with multiple-organ system dysfunction reflected in elevated illness severity scores and septic shock.166-173 ARDS associated with viral pneumonia has produced mixed results. NIV failure in SARS CoV-1 was markedly lower (30–33%)174-176 compared to influenza A/B (44%),173 H1N1 (59–85%),177-180 and MERS (92%).181 During COVID-19, a national database study reported NIV failure of 49%.125
Role of NIV in COVID-19
In China, where the initial treatment approach to COVID-19 favored NIV,11 an early nationwide study reported that NIV accounted for 87% of all mechanical ventilation with a substantially lower failure rate of 25% and an associated mortality rate of 17% (compared to 50% in those requiring invasive ventilation).121 A similar study from Wuhan also reported higher initial NIV usage (57%) with associated mortality of 41% versus 92% in patients requiring invasive ventilation.24
Specific NIV studies in COVID-19 largely focused on the use of CPAP in the non-ICU setting (Table 3).182-195 Unfortunately 46% of these were research letters and often lacked pertinent data.182-187 Nonetheless, 71% of all studies reported relatively low failure rates of 11–28% and relatively low associated mortality among those without care limitations (≤ 30%).153,182-184,186,190,192 This was accomplished mostly with moderate CPAP (≤ 12 cm H2O). However, these results were often accompanied either by low, vague thresholds for escalating care from low-level oxygen therapy (eg, supplemental O2 > 6 L/min to maintain > 92%)182,185 or provided no documentation whatsoever.184,187,195
Noninvasive Ventilation Usage and Outcomes
In 8 traditional observational studies, failure rates were 17–57% with associated mortality rates of 22–97%.188-195 In some studies, substantially higher mortality rates were reported in subjects in whom pre-NIV PaO2/FIO2 was < 150 mm Hg (53%)188 or subjects who had care limitations in place (55–72%).186,189,194
NIV duration was reported in 50% of studies with median values of 5–6 d.182,189 In some studies, median duration was 3–8 d when therapy was successful compared to 0.7–8 d in subjects who required intubation and 1.8 d in those with care limitations in place.184
Risk factors associated with NIV failure included increased age,185,188,189,194,195 admission Sequential Organ Failure Assessment (SOFA) score,184,192,195 Severe Acute Physiology Score (SAPS III),195 vasopressor use,195 renal replacement therapy,195 and number of comorbidities.189,192 Likewise, increased levels of C-reactive protein,186,188,194 interleukin-6,186 lactate dehydrogenase,189 d-dimers,185 and decreased platelet levels188 were also associated with NIV failure. Together these signify marked inflammation often observed in multiple-organ system dysfunction, endothelial dysfunction, pulmonary hypertension, and a procoagulant state.
Pulmonary-related variables associated with NIV failure included severity of pneumonia at hospital admission,186 decreased time to oxygen therapy failure (particularly when it resulted in PaO2/FIO2 < 150 mm Hg),189 and hyperpnea (ie, median minute ventilation of 15.8 L/min corresponding with median of 41.5 mm Hg).185 Despite the general association between low PaO2/FIO2 and NIV failure, some studies revealed that neither baseline values185 nor a cutoff of < 150 mm Hg were predictive.183 Nonetheless, larger studies affirmed the predictive value when PaO2/FIO2 was < 150 mm Hg.188,189 Successful NIV therapy was characterized by marked improvement in PaO2/FIO2 and decreased breathing frequency after initiation (particularly < 30 breaths/min) along with sustained  > 150 mm Hg over the course of therapy.189
> 150 mm Hg over the course of therapy.189
The characteristics of NIV use and outcomes in COVID-19-associated ARDS appear to be similar to those observed in non-COVID-19-associated ARDS in terms of the main drivers of therapeutic failure: (1) poor baseline oxygenation (and absence of sustained improvement with therapy), (2) comorbidities, and (3) illness severity and the presence of multiple-organ system dysfunction. The fact that several of these factors also drive mortality during invasive ventilation should be considered when judging the relative efficacy of either therapy.
Risk of Health Care Provider Cross-Infection During NIV
Only a few studies reported health care provider infection data.182,183,185,193 Two studies reported no infections when health care providers had access to the full range of personal protective equipment and when environmental controls were in place.183,185 Another study reported only that COVID-19 infection rates among health care providers increased from 6% to 10% after implementing NIV (the only detail provided was that bacterial filters were placed on the expiratory limb of the circuit).182 The most detailed information was provided by a study from Lombardy, Italy during the initial wave when hospital resources were extremely limited. Despite the availability of personal protective equipment, health care provider infection rate was high (11.5%) and corresponded to a lack of negative pressure rooms for conducting NIV therapy.193
During the 2003 SARS Co-V-1 pandemic, health care provider infection occurred primarily prior to identification of the highly contagious virus as the source and, therefore, prior to instituting protective measures.27,132,196,197 When health care providers were given access to the full range of personal protective equipment (along with stringent environmental controls), there was no further incidence of cross-infection.174,198
Summary
It was perhaps inevitable that COVID-19 would rekindle the long, contentious debate over what constitutes ARDS and its management. This issue dates back to the mid-1970s with Dr Petty’s “confessions of a lumper”1 and has continued throughout the history of ARDS, reflected in the need to develop a lung injury score,199 the American European Consensus Conference definition,200 and the Berlin definition.38 In the aftermath of COVID-19, it is quite possible that the definition of ARDS will be reexamined and perhaps modified to adjust for how specific viral pathogens might alter the progression of acute lung injury. The unanticipated pathophysiologic effects of the way in which SARS Co-V utilizes the ACE II receptor to infect pulmonary tissue stands as an important lesson to be incorporated into our understanding of ARDS.
In answer to the controversies that animated the early months of the pandemic, the vast majority of patients with COVID-19 who required invasive ventilation ultimately presented with ARDS. This is supported by its viral etiology, its histopathologic pattern and evolution, radiographic presentation and evolution, PEEP requirements, severity of hypoxemia, compliance, recruitment potential, duration of invasive ventilation, and responsiveness to NIV. All of these characteristics are uniformly consistent with non-COVID-19-associated ARDS. With regard to mortality associated with invasive ventilation in COVID-19, the majority of studies reported it to be within or below that reported in the general ARDS population.
Footnotes
- Correspondence: Richard H Kallet MSc RRT FAARC, 2070 Fell St #1, San Francisco, CA 94117. E-mail: richkallet{at}gmail.com
A version of this paper was presented at AARC Congress 2020 LIVE! held virtually, December 5, 2020.
Supplementary material related to this paper is available at http://www.rcjournal.com.
Mr Kallet has disclosed relationships with Nihon Kohden and ContinuED.
- Copyright © 2021 by Daedalus Enterprises
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.
- 42.
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.
- 48.
- 49.
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.
- 57.↵
- 58.↵
- 59.↵
- 60.
- 61.
- 62.
- 63.↵
- 64.
- 65.
- 66.
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.
- 109.
- 110.↵
- 111.
- 112.
- 113.↵
- 114.
- 115.↵
- 116.↵
- 117.↵
- 118.
- 119.
- 120.↵
- 121.↵
- 122.↵
- 123.
- 124.
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.↵
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.
- 136.↵
- 137.↵
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.↵
- 144.↵
- 145.↵
- 146.↵
- 147.↵
- 148.↵
- 149.↵
- 150.↵
- 151.↵
- 152.↵
- 153.↵
- 154.↵
- 155.↵
- 156.↵
- 157.↵
- 158.↵
- 159.
- 160.↵
- 161.↵
- 162.↵
- 163.↵
- 164.↵
- 165.↵
- 166.↵
- 167.↵
- 168.↵
- 169.↵
- 170.↵
- 171.
- 172.↵
- 173.↵
- 174.↵
- 175.
- 176.↵
- 177.↵
- 178.
- 179.
- 180.↵
- 181.↵
- 182.↵
- 183.↵
- 184.↵
- 185.↵
- 186.↵
- 187.↵
- 188.↵
- 189.↵
- 190.↵
- 191.
- 192.↵
- 193.↵
- 194.↵
- 195.↵
- 196.↵
- 197.↵
- 198.↵
- 199.↵
- 200.↵