Abstract
Mechanical insufflation-exsufflation (MI-E) is traditionally used in the neuromuscular population. There is growing interest of MI-E use in invasively ventilated critically ill adults. We aimed to map current evidence on MI-E use in invasively ventilated critically ill adults. Two authors independently searched electronic databases MEDLINE, Embase, and CINAHL via the Ovid platform; PROSPERO; Cochrane Library; ISI Web of Science; and International Clinical Trials Registry Platform between January 1990–April 2021. Inclusion criteria were (1) adult critically ill invasively ventilated subjects, (2) use of MI-E, (3) study design with original data, and (4) published from 1990 onward. Data were extracted by 2 authors independently using a bespoke extraction form. We used Mixed Methods Appraisal Tool to appraise risk of bias. Theoretical Domains Framework was used to interpret qualitative data. Of 3,090 citations identified, 28 citations were taken forward for data extraction. Main indications for MI-E use during invasive ventilation were presence of secretions and mucus plugging (13/28, 46%). Perceived contraindications related to use of high levels of positive pressure (18/28, 68%). Protocolized MI-E settings with a pressure of ±40 cm H2O were most commonly used, with detail on timing, flow, and frequency of prescription infrequently reported. Various outcomes were re-intubation rate, wet sputum weight, and pulmonary mechanics. Only 3 studies reported the occurrence of adverse events. From qualitative data, the main barrier to MI-E use in this subject group was lack of knowledge and skills. We concluded that there is little consistency in how MI-E is used and reported, and therefore, recommendations about best practices are not possible.
Introduction
Cough is an essential defense mechanism in clearing mucus from the airways. In invasively ventilated patients, cough is impaired due to an artificial airway as the vocal cords and glottis remain abducted.1,2 Sedation further exacerbates sputum retention as it limits the cough reflex, mucociliary clearance, and muscle strength. As a result, sputum retention in patients with an advanced airway is a common problem that may have substantial impact on ability to wean and to be extubated in the longer term.3
Airway clearance techniques are used by clinicians to mobilize and clear retained secretions. Endotracheal suctioning is most commonly used to remove secretions from the endotracheal tube (ETT), tracheostomy, and the upper airway.4 However, limitations to this technique include the inability to clear secretions from the lower airways and potential trauma to the upper airways.2
Mechanical insufflation-exsufflation (MI-E) is traditionally used in the neuromuscular population.5-7 It is conventionally used as a noninvasive device that delivers a positive-pressure breath to optimize tidal volume (VT) and lung recruitment and then quickly alternates to a negative-pressure breath. It is this rapid alternation between positive and negative-pressure breaths that augments gas flows, improves sputum mobilization, and ultimately stimulates a cough.6 More recently, there has been growing interest of MI-E use for intubated critically ill adults.7 Our research group has completed a number of practice surveys in Canada,8,9 the Netherlands,10 and the United Kingdom.11 These surveys illustrate the variable adoption of MI-E both nationally and internationally. Barriers to use cited in these surveys include limited clinician experience and knowledge of MI-E. Additionally, results illustrated MI-E use predominantly in the non-intubated critically ill subject group.8,9,11 The most frequently cited indication for MI-E use was the optimization of sputum clearance to prevent intubation or re-intubation.8-11 A Cochrane systematic review concluded that further research is required to establish the feasibility, efficacy, and safety of MI-E in the intubated population given the dearth of efficacy studies.12
The aim of this scoping review was to map current and emerging evidence on how MI-E is used in invasively ventilated critically ill adults. We sought spec-ific detail regarding the subject groups and stage of invasive ventilation for which MI-E as well as the practical application including pressures, times, and flows. We also sought to describe the outcomes and measures reported in MI-E studies as well as adverse events. This information will be used to inform research design in future MI-E studies.
Methods
Study Design
This scoping review followed the methods outlined by Arksey and O’Malley and advanced by other authors.13-15 The scoping review protocol has been previously published.16 There were no amendments made to the protocol during the conduct of the scoping review.
Study Identification
Our search strategy was a modified version of that previously used for the Cochrane systematic review of cough augmentation techniques in the critically ill.12 Modification required removal of terms used for airway clearance strategies other than MI-E. Furthermore, we did not exclude studies based on study design and did not restrict article selection based on language.16
The search criteria were applied between January 1990–April 2021 using electronic databases MEDLINE, Em-base, and CINAHL via the Ovid platform. PROSPERO and Cochrane Library were searched for relevant reviews, ISI Web of Science for conference abstracts, and the International Clinical Trials Registry Platform (trialsearch.who.int Accessed April 12, 2022) for unpublished and ongoing trials. The reference lists of relevant studies and reviews were examined to highlight any additional articles for inclusion.
Study Selection and Data Extraction
Criteria for inclusion of articles were (1) adult population with invasive ventilation via ETT or cuffed tracheostomy in an intensive care setting, (2) use of MI-E, (3) any study design with original data, and (4) published from 1990 onward. Citations were excluded if they included participants < 18 y or if they were editorial pieces, letters to the editor, and bench or animal-based studies.
Screening and data extraction were performed by 2 review authors (ES and WS) independently using a piloted data extraction form. Reviewers were responsible for contacting key authors for clarification of methods or additional data if required. Any disagreements during the review process were recorded and resolved by discussion or referred to a third reviewer (LR) for arbitration. EndNote X9 (Clarivate, Philadelphia, Pennsylvania) was used to manage citations.
Methodological Quality Assessment
The Mixed Methods Appraisal Tool17 was used to provide an assessment of study quality of full-text papers. Quality scores were not used to exclude studies. Citations of full publications only were scored by assigning quality scores 0–100% (0%, no criteria met; 100%, all criteria met) with 20% assigned per methodological criteria of which there were 5 per study design. Score ratings > 80% were classified as high quality, 80% moderate quality, and < 80% low quality.17 This process was completed independently by the reviewers (ES and WS) and then compared and discussed to generate consensus on ratings.
Results
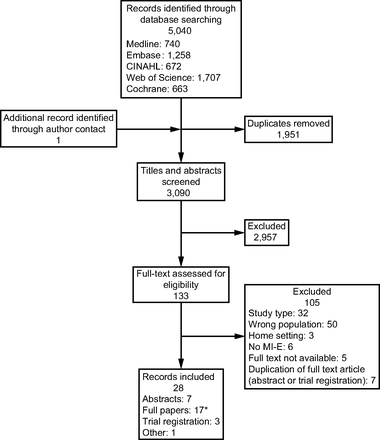
The initial search generated 3,090 unique citations. The full-text papers of 133 citations were assessed for eligibility. Once inclusion and exclusion criteria were applied, 34 citations representing 28 studies were taken forward for data extraction. One conference abstract was additionally highlighted through direct contact with an author. The search results are presented using a Preferred Reporting Items for Systematic Reviews and Meta-Analyses study flow diagram (Fig. 1).
Flow chart. *Full paper identified of 2 abstracts after closing date search. MI-E = mechanical insufflation-exsufflation.
Most studies (no. = 9) were randomized controlled trials (5 full-text publications,20-24 3 trial registrations,25-27 and one abstract 28) or descriptive studies (no. = 19) including observational cohort studies (no. = 7),29-35 surveys (no. = 6),8,10,11,36-38 and case study/series reports (no. = 5)39-43 and crossover trials (no. = 2).25,44 Studies were completed in 13 different countries. The Mixed Methods Appraisal Tool was completed for the 19 full-text publications. Only 5/19 (26%) studies scored 100% (high quality)8,10,11,23,29 (Table 1 and appendix 1, see related supplementary materials at http://www.rcjournal.com).
Study Characteristics
Population
Of the 28 studies, 20 studies provided information on the ICU population in which MI-E was studied (trial registrations no. = 3 and survey data no. = 5 excluded). Studies varied in terms of subject population with dissimilar reasons for intubation/invasive ventilation. The primary reason for intubation was recorded in 17/20 (85%) and was most commonly acute respiratory failure (no. = 12). Multiple underlying causes of acute respiratory failure were stated across studies including postoperative respiratory failure, pneumonia, cardiac arrest, acute spinal cord injury, and neuromuscular disease (NMD). Duration of invasive ventilation ranged from a minimum of 24 h to 10 d at the time of recruitment (Table 1).
Clinical Indications and Contraindications
We identified 10 different indications for use of MI-E. In clinical studies, the most commonly reported indication was presence of secretions and mucus plugging (9/28, 32%) followed by prophylactic airway clearance (7/28, 25%). Contraindications relating to concerns about using high levels of positive pressure (9/28, 32%) were most common. These findings were mirrored in survey reports of health care professionals (Table 2).
Reported Indications and Contraindications Mechanical Insufflation-Exsufflation
Clinical Studies
All 20 clinical studies reported on one or more elements of MI-E device settings. A range of devices were used; 11 (55%) reported using the E70 device and 2 (10%) the Emerson CoughAssist device. Eleven clinical studies did not specify device used. Twelve (60%) studies reported use via an ETT, 4 (20%) via tracheostomy, and 6 (40%) via a combination of ETT and tracheostomy.
A pressure setting combination of ± 40 cm H2O was most commonly used across reporting studies (10/20, 50%).21-24,26,28-30,39,44 Time settings were reported in 11/20 (55%) studies.21-24,29,30,34,39-41,44 Most commonly used time settings were inspiratory time 3 s, expiratory time 2 s, and 1 s pause. A pause duration was reported in 8/20 (40%) studies.20-24,30,34,44 Five studies (25%) reported use of one insufflation prior to an exsufflation breath (not reported in the remaining studies). Flow profile was specified in only 3 (15%) studies and was set at medium (no. = 2)20,28 or high (no. = 1).31 Use of oscillation was reported in 5/20 (25%) studies with 3/520,28,33 applying this option. One study applied an oscillation amplitude of 10 and frequency of 20 Hz,20 whereas only oscillation frequency was reported in the remaining 2 studies as high33 or 16 Hz. Treatment regimens varied across studies, with MI-E cycles being repeated up to every 20 min,29 hourly,32 1–2 times per day,34 3 times a day,22 4 times a day,43 and most commonly up to once per day.20,21,23,24,30,31,33,39,44 Five studies (25%) reported the inclusion of other treatment adjuncts along-side MI-E including side positioning,43 manual assisted cough,34 and suction.24,41,44 Table 3 provides an overview of described settings of MI-E use in invasively ventilated critically ill participants.
Detailed Overview of Mechanical Insufflation-Exsufflation Settings Across Studies
Seven (25%) studies described the individual applying MI-E. This was most commonly physiotherapists or respiratory therapists,22,23,30,34,41 followed by ICU nurses,22,29 caregivers/family,29,32 and ICU physicians.22
Outcomes and Measures
Of the 28 studies, 23 were appropriate to extract outcomes and measures; the remaining 5 were survey-based studies reporting on organization of care.
We identified 21 different outcomes measured in included studies (Table 4). Only 7 studies (7/23, 30%) clearly specified a primary outcome; these included aspirated/wet sputum weight,23,24 re-intubation rate,22 suction frequency,25 number of ventilator/ICU days,26 incidence of ventilator-associated pneumonia (VAP),34 and mortality rate in 1 year.27
Outcomes Measured*
Five (5/23, 22%) studies reported on one outcome only. These included cough peak flow (no. = 3),30,35,40 re-intubation rate (no. = 1), 43 and atelectasis resolution (no. = 1).39 Pulmonary mechanics was the most frequently reported outcome overall (no. = 9).21,23,24,29,31-33,42,44 These measurements encompassed measures of VT, minute ventilation, airway resistance, lung compliance, and vital capacity. Eight studies (8/23, 35%) reported on extubation failure/success;22,25-27,29,32,42,43 7 studies (7/23, 30%) reported on secretion clearance or wet sputum weight.21,23-25,31,33,44 Methods of outcome measurement varied across studies. Secretion clearance was primarily measured by aspirated sputum or sputum weight, most commonly at 5 min post-study intervention.23,44 When needed, 10 mL NaCl was used to rinse the suction catheter, and that weight was extracted from the result.23 Alternatively, secretion clearance was measured by frequency of endotracheal suctioning over a 24-h period.25 VAP incidence was measured throughout the period of intubation, with the frequency of assessment being unclear.20,25,34 The definition of VAP provided was “pneumonia in a patient who was on invasive ventilation for > 48 h.”34 Re-intubation rate or extubation failure was used as an outcome measure in 8 (8/23, 35%) studies and defined in 3/8 studies. Definitions of extubation failure varied across studies including 48 h following extubation,22 not needing a tracheostomy during hospitalization or at any time during follow-up,32 and discharge without re-intubation.29
Time points for measuring pulmonary mechanics were 5 min before and after the intervention and 1 h after the intervention. Cough peak flow was measured during and after intubation, mostly using the MI-E device.30,35,40
Adverse Events
Adverse events were addressed in 13/20 (65%) studies. For reporting purposes, we grouped adverse events into 3 commonly occurring categories, namely respiratory, hemodynamic, and other (Table 5).
Reporting of Adverse Events
Of the 13 studies, 10 studies reported no occurrence of adverse events in relation to MI-E. Three studies did report on the occurrence of adverse events.8,24,42 Documented adverse events included oxygen desaturation (< 85%),24 hemodynamic variation (increase or decrease of heart rate or blood pressure > 15–20% from baseline),8,24 re-intubation,42 pneumothorax,8,42 mucus plugging,8 hemoptysis,8 and chest pain.8
Barriers and Facilitators to MI-E Use
We found no qualitative studies to include in the scoping review; however, 3 survey studies reported qualitative data from open-ended questions.8,11,36 Themes illustrating barriers and facilitators to MI-E use were grouped under 6 of the 14 Theoretical Domains Framework domains: knowledge, skills, beliefs about consequences, intention, environmental context and resources, and social influences (Table 6). Barriers to MI-E use in the critically ill included the impact of team culture, a lack of clinical experience, and the need for additional resources and training with the device. Conversely, data illustrated positive intention to use the device with this subject group, with positive experiences described to date.
Reported Barriers and Facilitators to Mechanical Insufflation-Exsufflation Use
Discussion
In this scoping review, we mapped current and emerging evidence on MI-E use in invasively ventilated critically ill adults. We included 25 completed studies and 3 trial registrations published between January 1990–April 2021. Findings show that MI-E is predominantly used in ICU patients who have difficulties in weaning and sputum clearance. Studies predominantly investigated MI-E use in subjects with NMD and acute spinal cord injuries that does not reflect the heterogeneous nature of invasively ventilated critically ill adults. Perceived contraindications to MI-E use in the acutely intubated population related to the use of increased positive pressure. There was variation in MI-E device setup and the amount of details reported across studies. Only 3 studies reported on occurrence of adverse events. Qualitative data pertaining to subject and clinician experience of using MI-E in this subject group were lacking.
During invasive ventilation, positive-pressure breaths are delivered followed by a passive expiration. In contrast, MI-E delivers both positive- (insufflation) and negative- (exsufflation) pressure breaths. Therefore, it is noteworthy that we found the use of positive pressure to be a perceived contraindication, whereas negative pressure was not considered a contraindication or precaution for use of MI-E in invasively ventilated critically ill adults. In these patients, lung recruitment and de-recruitment are important considerations.45,46 Barotrauma and volutrauma associated with large VTs are well documented, and low-volume lung-protective ventilation is standard of care, particularly for patients with acute lung injury.45 However, de-recruitment of lung units can have an equally adverse impact on oxygenation and effective ventilation while attenuating lung injury.46 To date, no studies have examined the extent of de-recruitment or possible adverse events in relation to a negative-pressure exsufflation breath using MI-E.
Our review data indicate that MI-E is mainly studied with insufflation and exsufflation pressures of 40 cm H2O. The use of asymmetrical pressure settings and customization of pressure settings to endotracheal size have not yet been studied in invasively ventilated critically ill adults. Previous studies in an NMD non-ICU population47 illustrate that asymmetrical (ie, pressure settings to enhance the expiratory flow +30: −40 cm H2O) may enhance expiratory flow. One bench study examining the impact of an artificial airway on MI-E flows48 found higher pressures were required to overcome resistance to flow, particularly in narrower ETT sizes. Detail of flows, use of oscilla-tions, and timings were reported infrequently, which makes extrapolation of device setup into a clinical setting challenging. It is difficult to know whether these omissions are simply a lack of reporting detail or whether the full potential of MI-E settings was not used; this has been commented and queried previously.47 It should be acknowledged that advanced settings such as oscillations have not been avai-lable to clinicians for the duration of the data collection period; this may, therefore, have impacted on reporting of this feature. Data are needed to optimize the physiological impact of MI-E in invasively ventilated critically ill patients and to provide evidence-based guidance for our practice of care, training, and education.
We found multiple outcomes reported across studies including re-intubation rates, wet sputum weight, and respiratory parameters. The appropriateness of wet sputum weight as a primary outcome for examining the efficacy of MI-E is questionable.11,49 Although sputum clearance is important to quantify in invasively ventilated critically ill patients, a linear relationship does not exist between sputum quantity and disease severity.3 Consistency in the selection of outcome measures across MI-E studies would allow for meta-analyses, thus strengthening the overall evidence base. Development of a core outcome measure set, as recommended by the COMET Initiative (https://www.comet-initiative.org, Accessed September 2021), that specifically focuses on airway clearance in the invasively ventilated critically ill adult population is warranted.
Only 3 studies reporting occurrence of an adverse event including pneumothoraces, hemodynamic instability, and oxygen desaturation. Changes in hemodynamic parameters during MI-E were transient and did not require trial protocol cessation. Case reports of pneumothoraces have previously been described in an adult NMD non-ICU population50,51 following MI-E, although no causal relationship could be confirmed due to the use of MI-E.50-53
A common barrier to MI-E use was a perceived lack of skills and knowledge, suggesting an important opportunity for training and education. A European survey among ICU nurses showed that the knowledge related to respiration/ventilation was scored relatively low, although that would not be expected within this field of care.54 With MI-E being part of respiratory care, further qualitative inquiry to explore barriers and facilitators in greater detail could provide useful data to inform the optimal clinical implementation of research findings.
Strength and Limitations
Strengths of our scoping review are the use of systematic and transparent prespecified protocol, a search strategy with no methodological or language restrictions, appraisal of risk of bias using the Mixed Methods Appraisal Tool, and use of a theoretical framework to explore barriers and facilitators. We acknowledge that bench studies were excluded that may have provided additional data on MI-E settings in order to inform future research protocols.
Summary
This scoping review of MI-E use in invasively ventilated critically ill adults reports data on 28 studies. We conclude that there is little consistency in how MI-E is used and reported. This limits the strength of the overall body of evidence and the ability, therefore, to make recommendations about best practices. More studies are required, including more transparent reporting of device settings for the invasively ventilated critically ill patient. Additionally, we recommend development of a core outcome measure set for airway clearance in this population to promote consistency in outcome reporting in future intervention trials important to patients, clinicians, and researchers.
Footnotes
- Correspondence: Ema Swingwood MSc, Adult Therapy Services A804, Bristol Royal Infirmary, Upper Maudlin Street, Bristol, BS2 8HW, United Kingdom. E-mail: ema.swingwood{at}uwe.ac.uk
Supplementary material related to this paper is available at http://www.rcjournal.com.
The authors have disclosed no conflicts of interest.
Ms Swingwood holds a clinical doctoral research fellowship as funded by the National Institute of Health Research. Ms Stilma holds a personal (PhD fellowship) grant from NWO Netherlands Organization for Scientific Research.
Mss Swingwood and Stilma are joint primary authors.
- Copyright © 2022 by Daedalus Enterprises