Abstract
Multifocal cystic lung disease in infants is most commonly congenital, and is managed surgically with perioperative mechanical ventilation. Multifocal cystic lung disease in infants may be due to tuberculosis. We report a young infant with tubercular multifocal cystic lung disease and respiratory failure. The initial chest imaging revealed diffuse nodular infiltrates. Soon after admission he required conventional mechanical ventilation for respiratory failure. The bronchoalveolar lavage fluid grew Mycobacterium tuberculosis in culture. Subsequent chest imaging showed progression to multifocal cystic lung disease. The ventilation mode was changed to high-frequency oscillatory ventilation (HFOV) due to persistent CO2 retention in the presence of cystic lung disease. The cystic lung disease reversed with antitubercular treatment and prolonged HFOV with slow wean.
Introduction
Multifocal cystic lung disease is described in the literature as thin (< 4 mm) walled spaces involving multiple lobes of both lungs.1,2 Bronchogenic cyst and congenital adenomatoid malformations are the common congenital causes.1 Acquired lesions have been reported with chest trauma, lymphocytic interstitial pneumonitis, desquamative interstitial pneumonia, and infections.2 Tuberculosis (TB) has been reported to cause multifocal cystic lung disease in adults,3,4 though poor containment of initial infection usually presents with cavitatory (thick, > 4 mm walls) disease with a predilection for the posterior upper lobe.5 Affected children usually appear more ill, having severe cough, fevers, occasional night sweats, and weight loss. Some infants and young children with bronchial obstruction show signs of air trapping, such as localized wheezing or decreased breath sounds that may be accompanied by tachypnea or, rarely, respiratory distress. Respiratory failure requiring mechanical ventilation is uncommon,6 occurring in about 1.5–12.6% of patients hospitalized with TB,7–9 and has not been reported in adults with tubercular multifocal cystic lung disease.3,4 The average duration of mechanical ventilation for respiratory failure due to TB is 29 days.10 Respiratory failure from multifocal cystic or cavitatory disease in TB poses special challenges for mechanical ventilation, as treatment for TB extends over months.
We report a 2 month-old male infant who was diagnosed with disseminated TB with multifocal cystic lung disease after presenting with fever and fast breathing. To the best of our knowledge, this is the first pediatric patient ever reported with tubercular multifocal cystic lung disease and respiratory failure. Our institute's ethics committee waived the need for informed consent for reporting this case.
Case Report
A 2-month-old male infant (weight 4.5 kg) presented to the emergency department of a tertiary care hospital with fever and persistent tachypnea since the second week of life. Vital signs revealed respiratory rate of 118 breaths/min, heart rate of 140 beats/min, blood pressure of 96/60 mm Hg, oxygen saturation of 82%, and capillary refill time of < 3 seconds. On physical exam, he had severe respiratory distress with bronchial breath sounds and bilateral crepitations in the mammary, axillary, and infra-scapular areas. There was no stridor. The liver was palpable 3 cm below the costal margin, and the spleen 2 cm below costal margin. The arterial blood gas on admission revealed pH 7.228, PaCO2 48.4 mm Hg, PaO2 58.3 mm Hg, base excess –8 mEq/L, and arterial oxygen saturation 84.8%. The oxygen saturation improved to 98% when placed under oxygen hood. He was started on intravenous piperacillin-tazobactam and amikacin.
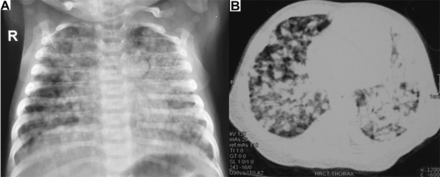
Chest radiograph and computed tomography scan of chest revealed diffuse nodular infiltrates (Fig. 1). The infant's earlier radiographs (done initially in the third week of life for tachypnea) showed similar findings, but he was lost to follow-up at that time. Complete blood count showed leukocytosis (15,900 leukocytes/mL3) with neutrophilic predominance (neutrophils 72%). The other laboratory values were normal. Screening for causes of immunodeficiency and TORCH (toxoplasmosis/other infection/rubella/cytomegalovirus/herpes simplex virus) infections was negative. Workup for TB revealed no reaction to purified protein derivative injection, and gastric aspirate was negative for acid fast bacilli. The infant's mother had been symptomatic for 5 months having fever, cough, and weight loss. Maternal chest radiograph was suggestive of TB. Hence, the diagnosis of disseminated TB was considered for the infant, and he was administered antitubercular drugs (rifampin 10 mg/kg, isoniazid 5 mg/kg, pyrazinamide 30 mg/kg, ethambutol 20 mg/kg) and dexamethasone (0.1 mg/kg/d in 4 divided doses).
Chest radiograph (A) and computed tomography (B) of chest showing diffuse nodular infiltrates in a 2-month-old male infant with tuberculosis.
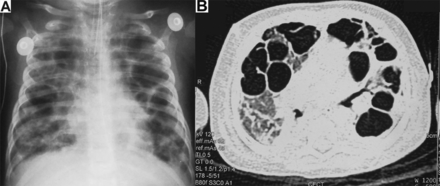
The infant's respiratory distress worsened on day 5 of admission, with subsequent respiratory arrest in hypoxic apnea, followed by cardiac arrest requiring intubation and cardiac compressions for 30 seconds. Mechanical ventilation was delivered (Servo-i, Maquet, Wayne, NJ) with pressure-controlled synchronized intermittent ventilation (SIMV) mode with pressure support. Initially, low ventilator settings were used; however, the settings had to be escalated over the next 2 weeks for increasing CO2 retention (Table). Imaging on day 8 showed progression to bilateral multifocal cystic disease in the lungs (Fig. 2). The bronchoalveolar lavage grew Mycobacterium tuberculosis in culture. He was continued on SIMV; however, on day 19 he had persistent CO2 retention (pH 7.229, PaCO2 92.3 mm Hg, PaO2 55.5 mm Hg, HCO3– 29.1 mEq/L, base excess 9.7 mEq/L, arterial oxygen saturation 81.5%). There was no evidence of gas trapping. Imaging showed bilateral multifocal cystic disease in lungs with no evidence of air leak. The ventilation mode was changed to high-frequency oscillatory ventilation (HFOV) delivered by an SLE 5000 (Specialized Laboratory Equipment, South Croydon, United Kingdom). He initially required high pressure amplitude of oscillation of 35 cm H2O, FIO2 of 0.70, mean airway pressure of 8 cm H2O, and frequency of 8 Hz for CO2 elimination. No recruitment maneuvers were performed. The settings were titrated to achieve adequate ventilation at low mean airway pressure of 8 cm H2O, with pressure amplitude of oscillation ranging from 20–29 cm H2O, and frequency 5–8 Hz. Oxygen saturation was targeted between 87–90% by pulse oximetry. Permissive hypercarbia with PaCO2 of 65 mm of Hg was accepted. No buffering strategy was used. After weaning the FIO2 to 0.40, the other settings of HFOV were weaned very slowly over 1 week to mean airway pressure of 8 cm H2O with pressure amplitude of oscillation of 20 cm H2O and frequency 6 Hz. The settings were kept lower than usual to avoid any air leak, in view of the bilateral multifocal cystic disease. These are lower than the usual settings at which the patients are shifted back to conventional ventilation in our unit. He was shifted to pressure SIMV on day 56, with peak inspiratory pressure of 15 cm H2O, PEEP of 5 cm H2O, and rate of 40 breaths/min. On day 71 he was extubated to nasal prongs. Repeat imaging showed resolution of the multifocal cystic lung disease.
Escalation of Settings of Conventional Mechanical Ventilation on Pressure-Controlled Synchronized Intermittent Mode
Chest radiograph (A) and computed tomography (B) showing multifocal cystic lung disease developing from nodular infiltrates in a 2-month-old male infant with tuberculosis on conventional mechanical ventilation.
The infant needed 3 fluid boluses (30 mL/kg total) and epinephrine infusion for 8 hours after the cardiopulmonary arrest. He did not need any more vasoactive drugs. He received 100 mL/kg of intravenous fluid in the initial week of hospital course. After that, feeds were started, and by 2 weeks he was on full nasogastric feeds and intravenous fluid was stopped. Later on his fluid volume was increased to 125 mL/kg/d to increase his caloric intake.
The infant received midazolam infusion (up to 4.6 μg/kg/min), fentanyl infusion (up to 2.8 μg/kg/h), as well as intermittent fentanyl (1 μg/kg every 4–6 h), and vecuronium infusion (1.4 μg/kg/min), and morphine infusion (20 μg/kg/h). They were used in various combinations, depending on the level of sedation and ventilator synchrony. Albuterol (200 μg via metered-dose inhaler) was used intermittently as needed. It was delivered using a holding chamber (AeroChamber, Monaghan Medical, Plattsburgh, New York). The endotracheal tube was suctioned every 4–6 hours as needed by trained pediatric ICU nursing professional.
The infant was transferred out of intensive care on day 82, with oxygen saturation of 95% on room air. He was subsequently discharged on day 84, on full nasogastric feeds and a steroid taper, and to complete 3 months of intensive phase antitubercular therapy, followed by 4 months of rifampin and isoniazid. The infant was doing well when evaluated 6 months later. He was on oral feeds, gaining weight, and had a respiratory rate of 55 breaths/min with oxygen saturation of 95% on room air. Developmental assessment was within normal limits and chest radiograph was normal.
Discussion
We have reported this young infant to highlight multiple unique aspects. Multifocal cystic lung disease (walls < 4 mm) is unusual in pulmonary TB, particularly in infants; they are more likely to have mediastinal or hilar lymphadenopathy with parenchymal disease. Clinical presentation in our patient was suggestive of congenitally acquired disease, in view of the early onset of symptoms, imaging showing nodular involvement of both lungs,11 and the mother being diagnosed with pulmonary TB. The extensive multifocal cystic lung disease could be secondary to congenitally acquired TB; mechanical ventilation may have contributed to the development of these cysts. The mild CO2 retention on admission and increasing CO2 retention prior to the development of the cysts suggest that small airway disease played a role in the formation of the cysts. It has been postulated that the granulomatous involvement of bronchioles may cause cystic lesions by a check-valve mechanism.3 This seems to be the likely mechanism in our patient, although interstitial air leak secondary to ventilation or dilated bronchioles with emptied out caseous material could also be possible, given the central and peripheral distribution of cysts.
Patients who have respiratory failure with multifocal cystic lung disease are at high risk of air leaks during mechanical ventilation, from rupture of the thin wall of the cysts. Our patient, in addition, developed hypercarbic respiratory failure on the conventional SIMV mode of mechanical ventilation, necessitating switch to HFOV.
HFOV has been used widely as a lung-protective mode of supportive gas exchange as rescue in patients who deteriorate on conventional mechanical ventilation. The most common indication for HFOV has been reported to be oxygenation failure.12 The use of HFOV in hypercarbic respiratory failure remains controversial, in particular if an underlying small airway disease is present. This is based upon the assumption that with this mode of ventilation the risk of dynamic air trapping is increased.13 In patients requiring rescue therapy with HFOV, the PaCO2 prior to HFOV has been reported to be higher in small airway disease patients, compared with diffuse airway disease, and returned to normal values after the initiation of HFOV.14 Several studies have reported the management of hypercarbic respiratory failure with HFOV. Frerichs et al reported that short-term (24 h) HFOV after failure of conventional mechanical ventilation for 72 hours in patients with COPD achieved more homogenous ventilation with effective CO2 elimination and oxygenation.15 Respiratory syncytial virus (RSV) induced respiratory failure with hypercarbia has been reported to be managed with HFOV using high mean airway pressure and large pressure swings.16 Duval et al described 3 different strategies in management of pediatric patients with HFOV17: the “open-lung” strategy, designed to rapidly recruit and maintain optimal lung volume in alveolar disease; the “low-volume” strategy in persistent air leak, where after an initial recruitment the mean airway pressure is reduced until the air leak ceases; and the “open-airway” strategy in obstructive airway disease, where mean airway pressure is used to recruit and stent the airways. Management of cystic lung disease with HFOV in our patient required a modified low volume strategy where HFOV was delivered with low mean airway pressure of 8 cm H2O for a prolonged duration to prevent air leak syndrome. To reduce air-trapping, lower frequencies were used to overcome the greater attenuation of the oscillatory waves in the cystic airways, permissive hypercapnia to enable reducing pressure swings as much as possible, and muscle paralysis to avoid spontaneous breathing. The active exhalation ability of the SLE 5000 oscillator most likely contributed to the CO2 elimination and further decreased the theoretical risk of dynamic air-trapping resulting from inadequate egress of air during passive expiration, as seen in high-frequency jet ventilation.
In neonates born with congenital cystic adenomatoid malformation, HFOV has been used for perioperative management of ventilation for 3–6 days.18 Our patient had respiratory failure with multifocal cystic disease due to an infectious agent, which required treatment over months. Prolonged HFOV over 37 days was thus delivered, in contrast with the usual mean duration of HFOV for 5–10 days.3,19 Our patient was also on further conventional mechanical ventilation for 15 days after HFOV, similar to the 8–22 days reported in clinical studies.19 Studies have shown no difference in inflammatory mediators between HFOV and conventional mechanical ventilation in premature infants and premature baboons.20,21 The role of HFOV versus conventional mechanical ventilation in causing and preventing reversible or permanent cystic lesions is unclear. Respiratory failure from potentially reversible multifocal cystic lung disease can be managed by prolonged HFOV with low mean airway pressures, low frequencies, very slow wean, and usual duration of conventional mechanical ventilation after HFOV.
Footnotes
- Correspondence: Rakesh Lodha MD, Department of Pediatrics, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India 110029.
Dr Mohari presented a version of this paper at the 6th World Congress on Pediatric Critical Care, held March 13–17, 2011, in Sydney, Australia.
The authors have disclosed no conflicts of interest.
- Copyright © 2012 by Daedalus Enterprises Inc.