Abstract
OBJECTIVE: To validate the hypothesis that fat tissue accumulation adjacent to the upper airway contributes to a predisposition to obstructive sleep apnea (OSA), irrespective of body mass index (BMI), as well as investigate the effect of the volume of fat tissue on pharyngeal mechanical loads.
METHODS: Fourteen subjects and 14 controls were enrolled in this study. Pharyngeal anatomy (the fat tissue volume in the retropalatal region and retroglossal region) were evaluated using magnetic resonance imaging. Whether the subjects had a segmental closing pressure higher than atmospheric pressure was determined by pharyngoscopy under general anesthesia. The difference in fat tissue distribution between subjects with OSA and BMI-matched controls was investigated. Fat tissue distributions in subjects with positive or negative segmental closing pressure were also compared.
RESULTS: Significant differences occurred between controls and subjects with OSA in volumes of parapharyngeal fat pad (P = .001), fat of soft palate (P = .01), as well as proportion of the parapharyngeal fat pad to the volume of total lateral pharyngeal soft tissues (P = .004). The volume of pharyngeal cavity, neck circumference, and volume of subcutaneous fat tissues were not significantly different statistically. Volume of fat in soft palate (odds ratio 5.893) and parapharyngeal fat pad in retropalatal and retroglossal region (odds ratios 1.781 and 1.845) were significant predictors of OSA. The volume of fat in the soft palate (P = .003) and parapharyngeal fat pad (P = .002) was higher in participants with positive retropalatal closing pressure; participants with positive retroglossal closing pressure had increased volumes of the tongue (P = .02) and the parapharyngeal fat pad (P = .004).
CONCLUSIONS: Patients with OSA have more fat tissue adjacent to the pharyngeal cavity than BMI-matched controls. Fats deposited around the upper airway may contribute to the collapsibility of retropalatal and retroglossal airway in both patients and controls.
Introduction
Obesity—particularly central adiposity—is a potent risk factor for obstructive sleep apnea (OSA). Weight gain as well as an increase in body mass index (BMI) has been associated with development of sleep apnea in large cohort studies.1 Previous studies have also suggested that visceral fat accumulation, neck circumference, and percentage of body fat are more important risk indicators for OSA in obese subjects.2,3
Obesity may be linked with sleep apnea through anatomic alterations in the upper airway (UA), decreases in lung volume, as well as effects on airway neuromuscular control.4 The anatomic alterations and mechanisms causing elevations in UA static mechanical loads in obesity are not well understood. It is difficult to explain why some obese subjects do not develop OSA with increased BMI. One possible mechanism is that only central adiposity constricts the pharyngeal cavity and contributes to pharyngeal collapsibility. That is, patients with OSA might have more fat tissue accumulation adjacent to the UA, independent of BMI. Some studies have found that the volume of pharyngeal fat tissue anterolateral to UA is associated with severity of OSA. An early study by Horner et al,5 using magnetic resonance imaging (MRI), showed a larger cross-sectional area of adipose tissue adjacent to the airway in obese subjects than weight-matched control subjects. A similar situation was reported by Mortimore et al6 in non-obese subjects with OSA. However, other studies have found that the parapharyngeal fat increase in obese subjects was not related to OSA and/or lateral dimension of pharyngeal cavity.7,8
The aim of this study was to investigate differences in fat tissue distribution between patients with OSA and BMI-matched controls, as well as to identify fat-associated risk factors for sleep apnea. Moreover, it also aimed to determine whether fat deposited around the retropalatal and retroglossal airway correlates to increased collapsibility of the corresponding pharyngeal segments.
QUICK LOOK
Current knowledge
Fat tissue accumulation adjacent to the upper airway contributes to the predisposition to obstructive sleep apnea, irrespective of body mass index.
What this paper contributes to our knowledge
Patients with obstructive sleep apnea have more fat tissue adjacent to the pharyngeal cavity than body mass index matched controls. Fat deposits around the upper airway may contribute to the collapsibility of retropalatal and retroglossal airway tissues.
Methods
The research protocol was approved by the ethics committee of Beijing Tongren Hospital. The aim and potential risks of the study were fully explained to each participant, all of whom provided written consent. The work was conducted in Beijing Tongren Hospital, Capital Medical University, Beijing, China.
Participants
All participants in this study were Chinese Han population. Subjects with previous pharyngeal surgery were excluded. A total of 28 participants were studied. All of the participants were recruited from in-patients of the department of Otolaryngology Head and Neck Surgery. All the subjects were undergoing surgery under general anesthesia.
All 14 subjects (11 males) with OSA underwent overnight polysomnography; their apnea and hypopnea index (AHI) scored > 5 events/hour (54.3 ± 24.1 events/hour, range 11.8–96.2 events/hour). All the subjects had symptoms suggestive of OSA (eg, any combination of sleepiness, snoring, witnessed apneas). These participants were age 39.4 ± 7.0 years (range 28.7–57.0 years) on average. Their BMI was 26.5 ± 2.9 kg/m2 (range 21.4–30.7 kg/m2), and the median of their Epworth Sleepiness Scale score was 11.0 (range 8.75–16.25).
All controls were non-snorers who did not feel sleepy during the day (Epworth Sleepiness Scale < 9). They did not have diseases relevant to the UA. The controls were included in the study if they matched subjects with sleep apnea for all 3 indices: age (± 10 years), BMI (± 1.5 kg/m2), and sex. Overnight polysomnography showed that their lowest SpO2 levels were ≥ 90% and AHI was < 5 events/hour (2.79 ± 1.24 events/hour, range 0.5–4.6 events/hour). The 14 controls (11 males) were age 42.8 ± 6.8 years (range 31.7–53.7 years) on average, and their BMI was 26.5 ± 3.0 kg/m2 (range 19.3–32.8 kg/m2).
The anatomical features of the UA were evaluated using MRI, then compared between subjects with OSA and BMI-matched controls. The effects of the anatomical risk factors on sleep apnea were analyzed. Subsequently the difference in the anatomical features associated with fat adiposity were compared between subjects with positive and negative retropalatal closing pressures (higher and lower segmental mechanical loads). Similarly, differences in fat distribution were also examined between subjects with positive and negative retroglossal closing pressures.
Neck Circumference
Neck circumference was measured at the level of the superior border of the cricothyroid membrane.
Polysomnography
Overnight polysomnography (Sandman, Tyco International, Ottawa, Ontario, Canada) was performed recording electroencephalography (C3/A2, C4/A1), electro-oculogram (ROC/A1, LOC/A2), submental electromyography, right and left anterior tibialis surface electromyography, electrocardiogram, nasal and oral air flow, and thoracic and abdominal movements. Pulse oximetry and plethysmography were assessed at the finger to evaluate oxygen saturation. Sleep stages and identification of apnea/hypopnea events were manually scored according to standard criteria.9 AHI was established as the number of apneas and of apneas/hypopneas per hour of sleep.
Magnetic Resonance Imaging of Upper Airway
UA imaging was performed in all participants during an awakened state using a 1.5-T MRI scanner (Signa Excite, General Electric, Madison, Wisconsin). The participant's head was in a supine position in the soft tissue Frankfort plane (tragus of the ear to orbital fissure), perpendicular to the scanner table, and fixed prior to the MRI.10
The following parameters were used during the scanning:
Sequential T1-weighted spin-echo axial sections, spanning from the most superior aspect of the nasopharynx to the false vocal cords (spin-echo repetition time (TR) = 500.0 milliseconds, echo time (TE) = 11.5 milliseconds, 320 × 192 matrix, 2 NEX (number of signal averages), FOV = 20.0 cm, slice thickness of 4.0 mm, and 0.0 mm skip)
Sagittal T1-weighted spin echo MR sequence, scanning the range from one mandibular rami to the other (TR = 640.0 ms, TE = 14.5 ms, 2 NEX, FOV = 20.0 cm, slice thickness of 4.0 mm and 0.0 mm skip, 320 × 224 matrix)
UA volumetric reconstructions were performed using an analysis system (Voxtool 3.0.64z, Volume Viewer, General Electric, Madison, Wisconsin). Fat volume was measured in each slice by mapping the adipose tissue compartments.
Image Analysis
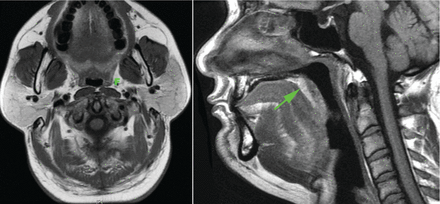
The analysis of images (Fig. 1) was performed by a researcher who was blinded to the case/control status and AHI of the subjects. The UA was divided into 3 parts: nasopharynx cavity (from the posterior aspect of the nasal fossa to the level of the hard palate), the retropalatal region (RP) (from the level of the hard palate to tip of the uvula), and the retroglossal region (RG) (from the tip of the uvula to the upper edge of the epiglottis).10,11 The 3 parts of the pharyngeal cavity and their surrounding soft tissues were evaluated separately, using the following measurements. The anatomical parameters for 3-dimensional volumetric measurements included: the airway in the RP, RG, and nasopharynx; the parapharyngeal fat pads; total subcutaneous fat tissues in RP and RG; fat tissues in the soft palate; the total soft tissues of the lateral pharyngeal wall, including muscles, parapharyngeal fat pads, palatine tonsils, mucosa and lymphatic tissues; and the tongue, soft palate, and tonsils.10 The component of lateral pharyngeal wall was evaluated based on the proportion of the parapharyngeal fat pad to the volume of total lateral pharyngeal soft tissues (%).
Parapharyngeal fat pad (F) and fat tissues in the soft palate (arrow in right panel).
Segmental Pharyngeal Mechanical Load
Segmental pharyngeal mechanical load in RP or RG was evaluated based on the closing pressure (Pclose) of the corresponding segment of the pharynx. Pclose was defined as the intraluminal pressure as the airway just closed (cross-sectional area = zero). A higher Pclose indicates a more collapsible pharynx.
To eliminate the influence of the neural control mechanism on UA collapsibility, a quick pharyngoscopy was performed to confirm the collapse of the pharyngeal segment in RP and RG, under general anesthesia with complete muscle paralysis.12
Each participant was placed in the supine position, with the neck in a neutral position. SpO2, electrocardiogram, and blood pressure of the subjects were monitored. The subjects were medicated with 0.5 mg of atropine sulfate and 1–2 mg of midazolam and 0.02–0.1 μg/kg of sufentanil. A fiberoptic pharyngoscope (P4, Olympus, Tokyo, Japan) was passed through the naris to the pharyngeal segment of RG. Then general anesthesia was administered using propofol (0.5 mg/kg) and vecuronium (0.08 mg/kg) (administered intravenously). After complete paralysis was produced, the pharyngeal image at the level of RG was recorded. The pharyngoscope was then quickly withdrawn to the level of RP to get the pharyngeal images, which completed the examination. The procedure of pharyngoscopy took between 8–15 seconds. A modified mask was used to maintain ventilation if the examination was prolonged. SpO2 remained ≥ 99% throughout this test in all subjects.
If any level of the pharyngeal segment had an area = 0 (ie, total collapse), the corresponding segmental Pclose was judged as higher than atmospheric pressure (ie, positive). If the pharyngeal segment remained patent, the segmental closing pressure was judged to be negative.
According to the segmental closing pressure in the retropalatal area (Pclose in RP), participants were divided into a high RP mechanical load group (Pclose > atmospheric pressure) and a low RP mechanical load group (Pclose ≤ atmospheric pressure). Similarly, participants could be divided into a high retroglossal mechanical load group and a low RG mechanical load group according to the segmental closing pressure in RG.
Analysis
The number of observations needed to compare 2 means of cases and controls was estimated13 by the formula:
The values of σ and δ were estimated by the data of the pre-test (n = 7 for each group).The sample size was determined by providing a 90% chance of rejecting the hypothesis of no difference at the .05 level of significance (Z2α = 1.96). The data from the following parameters were used for calculating sample sizes: volume of parapharyngeal fat pad in RP/RG, volume of fat in soft palate, and volume of total fat tissues in RP. The minimum sample size was 10 subjects for each group.
A single-sample Kolmogorov-Smirnov test was used to test whether the variables were normally distributed (SPSS 13.5, SPSS, Chicago, Illinois). Descriptive statistics were presented as means ± SD. Controls and subjects were compared using paired samples t tests. Participants with positive or negative closing pressures were compared using independent sample t tests; the association between parameters was evaluated using the Spearman correlation coefficient. Logistic regression analysis was used to identify the factors associated with OSA. An enter selection procedure was used to identify the effect of anatomical variables, age, and BMI. The effects of variables are expressed as adjusted odds ratios with 95% confidence intervals and associated P values. P < .05 was considered significant. Effects of BMI and age were assessed at each procedure, but no significant effects were found.
Results
Anthropometric Measurements
Apnea and hypopnea index, BMI, age of the subjects, as well as all the anatomy parameters in both groups were normally distributed (P > .05). No significant differences emerged in BMI (P = . 99) when comparing controls with subjects with OSA. No significant differences in age existed between the groups (P = .20).
Pharyngeal Closing Pressures
Eight controls (57.1%) and 2 subjects (14.2%) had negative Pclose in RP, whereas 9 controls (64.3%) and 1 subject (7.1%) had negative Pclose in RG.
Fat Tissue Distribution in Subjects With OSA, Compared to Matched Control Subjects
Comparison of anatomical parameters in subjects with OSA and controls are listed in Table 1. Significant differences emerged in volumes of parapharyngeal fat pad and fat in soft palate between controls and subjects with OSA. Table 2 provides the age and BMI adjusted odds ratios for association between anatomical risk factors and sleep apnea.
Comparison of Anatomical Parameters in Subjects With Obstructive Sleep Apnea and Controls
Adjusted Odds Ratios for Association Between Anatomical Risk Factors and Sleep Apnea
Figure 2 demonstrate the volume of fat tissues in the retropalatal region in subjects and controls with similar BMI values. More volume of fat tissues in RP appeared in subjects with sleep apnea than those in controls at a similar BMI. The correlation between BMI and volume of UA fat tissues in retropalatal region was weak (r = 0.384, P = .044).
Scatter plot of volume of upper airway fat tissues in retropalatal region (parapharyngeal fat pad in retropalatal region in addition to fat in soft palate) and body mass index. Note: the volume of fat tissues in the retropalatal region in subjects with sleep apnea (squares) was more than those in controls (dots) at the same value of body mass index.
Fat Tissue Distribution and Collapsibility of Pharynx in RP and RG
A comparison of fat tissue distribution in participants with positive or negative closing pressure in the retropalatal area is provided in Table 3. BMI as well as volumes of the fat in the soft palate and the parapharyngeal fat pad in RP was higher in subjects with positive retropalatal closing pressure. Meanwhile, fat-free tissues of the lateral wall in RG and neck circumference did not significantly differ between groups.
Comparison of Anatomical Parameters in Subjects With Positive or Negative Closing Pressure in Retropalatal Area
A sub-analysis was conducted to compare fat tissue distribution in controls with positive (n = 6) or negative closing pressure (n = 8) in RP. The result showed increased fat component in the soft palate in positive closing pressure subgroup (2.83 ± 0.92 cm3 vs 1.66 ± 0.70 cm3, P = .02). While the volume of parapharyngeal fat pad in the retropalatal region did not show any difference between the 2 subgroups (8.22 ± 2.68 cm3 vs 6.02 ± 2.82 cm3, P = .17).
Table 4 summarizes the comparison of fat tissue distribution in participants with positive or negative closing pressure in RG. Significant differences emerged in regard to volume of parapharyngeal fat pad in RG between positive and negative closing pressure groups. However, BMI and neck circumference did not significantly differ between groups.
Comparison of Anatomical Parameters in Subjects With Positive and Negative Closing Pressure in Retroglossal Area
Comparison of parapharyngeal fat pad in RG in controls with positive (n = 5) or negative closing pressure (n = 9) in retroglossal area did not show significant differences (3.84 ± 1.37 cm3 vs 2.69 ± 1.91 cm3, P = .26).
Discussion
Central Fat Distribution and OSA
The current study found a disparity in the relationship between obesity and UA adipose tissue volume in subjects with OSA. More fat tissue accumulation adjacent to the UA was observed in subjects, compared to BMI-matched controls. The central fat distribution that predisposes to OSA may occur at a lower level of BMI.
Some of the previous studies in white subjects supported the finding that the central obesity type and increased parapharyngeal fat pads are associated with the degree of OSA5,6 However, other studies failed to find the correlation between parapharyngeal fat and AHI.7 Such discrepancies may arise from different study populations or sampling strategies. Another possible reason is that the contributions of fat deposits in the UA region to the development of OSA are different among individuals/populations. Other anatomical and non-anatomical risk factors, such as enlarged tonsils, decreased volume of bony enclosure of UA, and dysfunction of respiratory control, also contribute to AHI. Different levels of these risk factors among subjects may obscure the correlation between the volume of parapharyngeal fat and AHI.
All the subjects of this study were Chinese Han population. Ethnic differences in OSA have been mentioned in multiple studies,14,15 Contribution of obesity to sleep apnea manifests differently in diverse ethnic groups. First, the BMI value of most studies in Asian patients with sleep apnea, as well as this studied population, was lower, compared with the typical profile of OSA patients in a white population. Asian men were more likely to have severe OSA at a non-obese level of BMI, when using white standards for obesity. Furthermore, the odds ratio for OSA per one standard deviation increase of BMI was greater in the study in whites, compared with odds ratios from the Hong Kong Chinese population.16 However, there are few data sets about central fat distribution in patients with OSA in Asian populations.
The pathogenesis of central obesity and deposition of fat in patients with OSA is not clear. It is suggested that intermittent hypoxia and stress caused by sleep apnea may add to inflammatory responses and dysregulation of glucose and lipid metabolism,16,17 consequently causing deteriorated obesity.
It appears that the volume of fat tissue enclosed by the mandibular ramus and vertebra was most likely to contribute to sleep apnea, specifically parapharyngeal fat pads and the fat tissues deposited in the soft palate. It is reported18 that fat deposited in the 2 spatia veli palate (which is located between the muscle of palato-uvularis and the lower margin of both tensor palati and levator palate) is evidently increased in OSA patients. Improved sleep apnea can be achieved by removing fat tissues in soft palate and widening airway size by surgery.19,20 There was small relationship between the volume of deep fat and the neck circumference, which was more affected by the circumferential subcutaneous fat. The latter may be affected by BMI, but appear less important in the pathogeneses of OSA. It is possible that increased volume of the deep fat tissues adjacent to the UA causes increased pharyngeal mechanical loads and, consequently, sleep apnea.
Mechanical Effects of Adiposity Around the Pharynx
What are the mechanical effects of adiposity around the pharynx? According to previous studies,20 one possible mechanism is that fats deposited around the UA constrict the pharyngeal cavity. Welch et al21 investigated fat tissue and UA volume in the neck during a weight-loss program, and found an increase in the UA volume in both the RP and RG regions. However, in Hora et al's study,22 despite the lateral compression of the pharyngeal cavity, increased fat pad thickness was not associated with the transverse dimension of the airway, which was an independent predictor of OSA. Volume of the pharyngeal cavity did not significantly differ, statistically speaking, in matched-BMI controls and subjects, even when subjects had larger fat pads than in the current study. Thus, we speculated that the difference in airway collapsibility between controls and subjects could not simply be explained by the narrowing of the pharyngeal cavity.
Larger fat tissue volume in RP may be associated with positive segmental closing pressures. There was no significant difference (positive or negative Pclose) in the fat-free soft tissue of the lateral pharyngeal wall. These results indicate that increased fat accumulation in the RP region might contribute more to collapsibility of the retropalatal pharynx. However, in the RG area, other anatomical deficiencies (eg, hypertrophy of the tongue and fat-free tissues of the lateral wall) also appear to be important factors that added to the collapsibility of the airway. Also, fat tissues deposited in the lateral wall of RG may add to the volume of total collapsible soft tissues in the corresponding segment of airway, which seemed to be predisposed to increased retroglossal collapsibility.
It appears that the controls with positive closing pressures in RP had increased volume of fat tissues in the soft palate, despite small sampling size. The result of sub-analysis indicated that, even in non-snoring controls, increased fat tissue may be associated with increased UA collapsibility, which may act as a risk factor in the development of sleep apnea. Sleep apnea might develop if dysfunction of UA dilators compensation exists. Although the mean value of parapharyngeal fat pad in RP and RG was higher in the positive closing pressure group, the P value was > .05. The differences might be more significant with a larger sample size in further investigations.
Limitation of the Study
First, this study assessed only whether Pclose at the RP or RG segment was higher than atmospheric pressure. The correlation of closing pressures with anatomical parameters could be achieved more accurately if the segmental mechanical loads were measured quantitatively by curve fitting. Moreover, the relationship of fat tissue distribution and the severity of sleep apnea was not discussed in this study, for limitation of the sample size. Further studies are needed to investigate the influence of (1) adiposity around the pharynx on the severity of sleep apnea, and (2) the central adiposity in patients with sleep apnea.
Conclusions
In conclusion, patients with OSA have more fat tissue adjacent to the pharyngeal cavity than do sex-, age-, and BMI-typed control subjects. We also found that fats deposited around the UA added to the volume of total collapsible soft tissues, which were correlated to increased retropalatal and retroglossal collapsibility, as evident in both patients with OSA and the control groups.
Footnotes
- Correspondence: Demin Han MD PhD and Jingying Ye MD PhD, Department of Otolaryngology Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University. 1 Dongjiaominxiang Street, Beijing, 100730, China. E-mail: handm{at}trhos.com; yejingying{at}yeah.net.
This research was partly supported by Chinese National Scientific Fund grants 30672304 and 30973295, Beijing Municipal Commission of Education grant KZ201010025020, and Science and Technology Key Projects grant KZ201010025020. The authors have disclosed no conflicts of interest.
- Copyright © 2012 by Daedalus Enterprises Inc.