Abstract
BACKGROUND: The significance of changes in PaCO2 during long-term noninvasive ventilation (NIV) on prognosis remains unclear. We aimed to clarify whether stabilizing PaCO2 during NIV had a favorable prognostic effect.
METHODS: Data from 190 subjects with restrictive thoracic disease and who received long-term NIV were studied retrospectively. The annual change in PaCO2 during NIV was determined using a simple linear regression method for each subject who had at least 4 6-month intervals of PaCO2 data. Annual changes in PaCO2 during long-term NIV and possible confounders were analyzed with discontinuation of long-term NIV as the main outcome.
RESULTS: One hundred and twenty-five subjects who had > 4 6-month intervals of PaCO2 data were included in the study. PaCO2 during long-term NIV decreased in 41 subjects (group 1; < 0 mm Hg/y), increased slightly in 42 subjects (group 2; between 0 and 1.85 mm Hg/y), and increased significantly in 42 subjects (group 3; > 1.85 mm Hg/y). Smaller annual changes in PaCO2 (P < .001) and a control ventilator mode (P = .008) were associated with a significantly higher probability of continuing NIV, compared with decreased PaCO2 3–6 months after the start of long-term NIV (P = .11). The 10-y probability of continuing NIV was 69% in group 1, 39% in group 2, and 12% in group 3.
CONCLUSIONS: A decrease in the annual change of PaCO2 during long-term NIV was shown to be a significantly prognostically favorable factor. Efforts to reduce PaCO2 should be made if PaCO2 increases at a greater rate during long-term NIV.
- chronic respiratory failure
- hypercapnia
- home mechanical ventilation
- noninvasive ventilation
- PCO2
- restrictive thoracic disease
Introduction
Persistent CO2 retention in subjects with chronic respiratory failure may reflect an adaptive process that allows a lower level of alveolar ventilation, thus unburdening the respiratory muscles and reducing breathlessness, particularly when long-term oxygen therapy is prescribed.1,2 This compensatory process may demonstrate the wisdom of nature2; however, ventilatory support, such as noninvasive ventilation (NIV), should be applied if hypercapnic ventilatory failure develops despite this compensation.3–9 Over the last 2 decades, domiciliary NIV has been widely used to treat subjects with chronic hypercapnic respiratory failure.10–16 Improved daytime PaCO2 after the initiation of NIV has been correlated with a change in PaCO2 while receiving nighttime NIV,6,17 and the most important mechanism underlying the long-term reduction in daytime PaCO2 is an augmented ventilatory response to CO2.3–5 Arterial blood gases (ABGs) stabilize a few months after NIV initiation, and are sustained for many years in subjects with both COPD10,18 and restrictive thoracic disease.10,12,13,19,20
We previously showed that the average PaCO2 of subjects breathing spontaneously decreases rapidly within several weeks of NIV, and thereafter increases gradually throughout long-term NIV.18,20 We hypothesized that the adaptive mechanism that permits chronic hypercapnia occurs even in subjects receiving long-term NIV. In this study, we retrospectively reviewed PaCO2 data sampled at 6-month intervals and at clinically stable times for > 2 y, and calculated the rate at which PaCO2 changed in each subject.
Generally in nature, adaptations occur during disadvantageous situations. Therefore, we hypothesized that subjects with a greater annual change in PaCO2 would have lower continuation rates of NIV than those with a more stable PaCO2. Thus, we aimed to clarify which pattern of PaCO2 change during long-term NIV would be suitable for continued use of NIV.
QUICK LOOK
Current knowledge
Persistent hypercapnia in chronic respiratory failure results in adaptation and a reduction in the required minute ventilation, reducing the work of breathing and dyspnea. Noninvasive ventilation (NIV) has been used to reduce daytime hypercapnia and improve comfort in patients with chronic lung disease.
What this paper contributes to our knowledge
During long-term NIV, a reduction in arterial carbon dioxide over 3–6 months is associated with improved compliance with continued NIV use. In patients with progressive hypercapnia despite NIV, efforts to control arterial carbon dioxide are warranted.
Methods
Subjects
All subjects with restrictive thoracic disease who had begun NIV at the National Hospital Organization Minami-Kyoto Hospital (Kyoto, Japan), the National Tokyo Hospital (Tokyo, Japan), or the Kyoto University Hospital (Kyoto, Japan), from June 15, 1990 to August 2, 2007, were included in this study. This same subject cohort was assessed in our previous studies.16,20 All subjects had chronic hypercapnic respiratory failure. The criteria to initiate NIV were based on medical symptoms with lasting diurnal hypercapnia (PaCO2 > 45 mm Hg) and/or nighttime hypoventilation and/or clinical instability with frequent hospitalizations. Subjects with other diseases such as neuromuscular disorders, obesity hypoventilation syndrome, bronchiectasis, or COPD were excluded. The subjects were followed up to November 30, 2007.
This study was performed in the Department of Respiratory Medicine, National Hospital Organization Minami-Kyoto Hospital, Kyoto; the Department of Respiratory Medicine, National Tokyo Hospital, Tokyo; and the Department of Respiratory Care and Sleep Control Medicine, Kyoto University Graduate School of Medicine, Kyoto, Japan. This study was approved by the ethics committee of Kyoto University. According to the advice of the ethics committee of Kyoto University, the study procedure was disclosed on our institution website, and all questions have been answered.
Measurements
Data on age at the initiation of NIV, gender, body mass index, preexisting comorbidities (Charlson index), existence of lung lesions, vital capacity (percentage of predicted), FEV1 over forced vital capacity (FEV1/FVC), length of long-term oxygen therapy before the initiation of NIV, clinical status at the start of NIV (ie, acute or chronic), concurrent usage of long-term oxygen therapy after the initiation of NIV, ventilator mode (assisted or controlled mode), inspiratory positive airway pressure, expiratory positive airway pressure, pressure support level (inspiratory positive airway pressure – expiratory positive airway pressure), breathing frequency, PaCO2 after 3–6 months of NIV, and annual changes in PaCO2 during long-term NIV were all surveyed and assessed to clarify the risk factors.
Data on daytime 6-month intervals of ABGs were gathered from 12 months before the initiation of NIV to the observation end point, if obtainable. ABGs were obtained primarily from 9:00 am to 1:00 pm, with the subject in the supine position and spontaneously breathing either room air or prescribed oxygen. ABGs were sampled in subjects who were in a stable situation without exacerbation.
All data on the medical course of the subjects were gathered from the medical records.
Clinical Procedure for Initiating Long-Term NIV and Follow-Up
The detailed procedure for initiating long-term NIV has been described elsewhere.16 On initiation of NIV, volume preset ventilators were applied with either custom-made nasal masks21 or commercial interfaces. Pressure preset ventilators using bi-level positive airway pressure appliances were utilized with commercial masks. Oxygen was supplemented to keep an arterial oxygen saturation > 95% during daytime NIV and > 90% during nighttime NIV.
During follow-up, all subjects who were on long-term NIV and/or long-term oxygen therapy had to visit an out-patient clinic once a month, as this is compulsory in Japan. Chest x-rays and ABG analyses were routinely carried out every 3–6 months.
Statistical Analysis
The change in PaCO2 during NIV was determined using a simple linear regression method for each subject who had at least 4 6-month intervals of PaCO2 data from 6 to 96 months after the start of long-term NIV. The subjects were divided into 3 groups using tertiles of the distribution of the changes in PaCO2 (group 1, < 0 mm Hg/y, n = 41; group 2, from 0 to 1.85 mm Hg/y, n = 42; group 3, > 1.85 mm Hg/y, n = 42).
Means ± SD were used for subject features and ventilator settings. One-way factorial analysis of variance and post hoc analysis (Scheffé test) were employed for continuous variables, chi-square tests were used, and the 2 × 3 contingency table for categorical variables was employed to evaluate differences in subject features among the 3 groups. Continuance of long-term NIV relative to risk factors, including the annual change in PaCO2, was assessed by univariate Cox proportional hazards regression analysis. Thereafter, continuance was evaluated using multivariate analysis with only significant risk factors (P < .05) in univariate analysis. Continuance of long-term NIV was also estimated by Kaplan-Meier analysis (log-rank test). To assess differences in PaCO2 over time among the 3 groups, repeated-measures analysis of variance and post hoc analysis (Scheffé test) were applied. One-way factorial analysis of variance was used to assess differences in PaCO2 at the same time points among the groups.
Results
Subject Characteristics
There were 190 subjects who were on NIV for > 2 years and were available for follow-up. Two subjects ceased NIV within 2 y due to recovery from hypercapnic respiratory failure. Twenty-one subjects died within 2 y of NIV. Twenty-five subjects were on NIV for < 2 y but were still on NIV at the final follow-up time point (November 30, 2007). Seventeen subjects continued NIV > 2 y but had < 4 6-month intervals of PaCO2 data. Thus, 125 subjects were available for the final analysis (Fig. 1).
Of the 190 subjects available for follow-up, 2 discontinued noninvasive ventilation (NIV) within 2 y due to improvement, 21 died within 2 y of beginning NIV, 25 used NIV < 2 y, and 17 continued NIV > 2 y but had < 4 6-month intervals of PaCO2 data. Thus, 125 subjects were available for the final analysis.
Of the 125 subjects, 119 were post-tuberculosis, 4 had kyphoscoliosis, 1 was post-hemothorax, and 1 had pneumoconiosis. Almost all post-tuberculosis subjects with pulmonary lesions received surgical treatment such as thoracoplasty and/or artificial pneumothorax and/or lobectomy. Post-tuberculosis subjects without pulmonary lesions were diagnosed as having Pott disease.
Of 125 subjects, 62 discontinued long-term NIV. Forty-eight subjects died during long-term NIV, 7 of whom did not receive NIV in the last few days (mostly due to delirium) and received only oxygen therapy. Two subjects received endotracheal invasive ventilation and died with severe exacerbations. Twelve subjects were switched to tracheostomy positive-pressure ventilation due to disease progression, 10 of whom died during long-term tracheostomy positive-pressure ventilation, and 2 continued long-term tracheostomy positive-pressure ventilation until November 30, 2007.
Subject characteristics relative to their annual changes in PaCO2 are shown in Table 1. On the whole, subjects were characterized by serious restrictive ventilatory failure, crucial hypercapnia, slight malnutrition, and unstable clinical status. Some subjects had a mild obstructive ventilatory defect; however, their chest x-rays and/or chest computed tomographs did not indicate typical emphysema. More subjects in group 1 had no pulmonary lesions compared with groups 2 and 3. Among subjects using bi-level positive airway pressure devices, the expiratory positive airway pressure values were slightly lower in the subjects in group 3 compared with those in group 1. For inspiratory positive airway pressure and pressure support level, there was no statistical difference between the 3 groups. After 3–6 months of NIV, there was no significant difference in PaCO2 values among the 3 groups.
Subject Characteristics at the Beginning of Long-Term Noninvasive Ventilation
The Annual Change in PaCO2 During Long-Term NIV
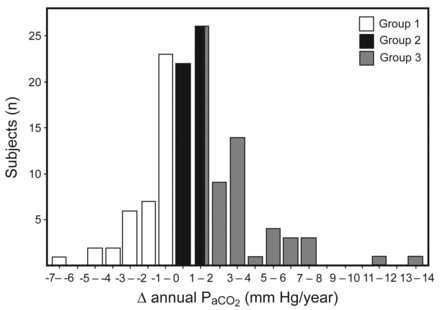
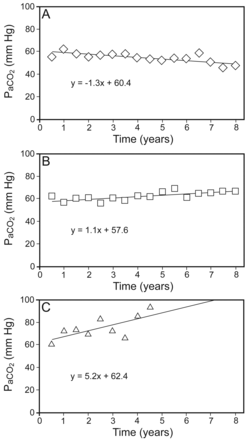
The distribution of the annual changes in PaCO2 is shown in Figure 2 and the 3 typical PaCO2 patterns are presented in Figure 3. In group 1, the PaCO2 rate decreased throughout long-term NIV. In group 2, PaCO2 increased slightly during long-term NIV. In group 3, PaCO2 increased greatly during long-term NIV.
The distribution of annual Δ in PaCO2. A total of 125 subjects were divided into three groups based on their annual ΔPaCO2. Group 1 had ΔPaCO2 < 0 mm Hg/y (n = 41); group 2 had ΔPaCO2 0–1.85 mm Hg/y (n = 42); and group 3 had ΔPaCO2 > 1.85 mm Hg/y (n = 42).
Three representative patterns of PaCO2 changes during long-term noninvasive ventilation (NIV). A: PaCO2 decreased in the subjects from group 1. B: PaCO2 increased slowly in group 2. C: PaCO2 increased rapidly in group 3.
Time Course of PaCO2 Before and After NIV in the 3 Groups
An analysis of the PaCO2 time course in the 3 groups is shown in Figure 4. There was a significant difference in the PaCO2 time course among the 3 groups (P = .013). In the post hoc analysis, significant differences were observed between groups 1 and 2 (P < .001), groups 1 and 3 (P < .001), and groups 2 and 3 (P < .001).
The PaCO2 time course during long-term noninvasive ventilation (NIV). A comparison of the subjects grouped according to their annual change in PaCO2 is shown. Data are presented as mean ± SD. There was a significant difference in PaCO2 over time among the three groups (P = .013). Post hoc analysis showed significant differences between groups. Group 3 had P < .001 compared with group 1 and P < .001 compared with group 2; group 2 had P < .001 compared with group 1.
There were no significant differences in PaCO2 from the 12th month before NIV was initiated to the 24th month after the start of NIV in the 3 groups. From the 30th to the 98th month of long-term NIV, there were significant differences in PaCO2 at the same time points among the 3 groups (Fig. 4).
Comparison of the Continuation Rate of Long-Term NIV
In the univariate analysis, being female, of a younger age at the beginning of NIV, having no parenchymal pulmonary lesions, a controlled mode, relatively low PaCO2 levels after 3–6 months of NIV, and lower annual changes in PaCO2 during long-term NIV were all significantly associated with higher continuation rates of NIV (Table 2). Ventilator settings such as inspiratory positive airway pressure, expiratory positive airway pressure, pressure support level, and breathing frequency appeared to have no bearing on the continuation rates of NIV.
Comparisons of Continuation Rates of Long-Term Noninvasive Ventilation by Several Risk Factors Including Annual Changes in PaCO2 (Univariate Modality Model)
Multivariate analysis showed that lower annual changes in PaCO2 during long-term NIV (P < .001) and controlled ventilation (P = .008) were both significantly related to higher continuation rates of NIV (Table 3). There was no statistical difference in continuation rates between groups 1 and 2 (P = .89).
Comparisons of Continuation Rates of Long-Term NIV by Several Risk Factors Including Annual Changes in PaCO2 (Multivariate Modality Model)
Results of the Kaplan-Meier analysis revealed significantly enhanced continuance of long-term NIV in subjects with lower annual changes in PaCO2 (P < .001, Fig. 5). The 5- and 10-y probabilities of continuing NIV were, respectively, 75.2% and 69.0% for group 1, 79.9% and 38.9% for group 2, and 58.2% and 11.7% for group 3.
Kaplan-Meier curves of the continuation rates of long-term noninvasive ventilation (NIV). Subjects were divided into three groups according to their annual changes in PaCO2. Subjects with lower annual changes in PaCO2 had a significantly better prognosis (log-rank test, P < .001). The 10-y probability of continuing NIV was 69% for group 1, 39% for group 2, and 12% for group 3.
Discussion
This study showed that, after the initiation of long-term NIV, PaCO2 decreased gradually in one third of subjects, increased slowly in one third of subjects, and increased greatly in one third of subjects. Those subjects with smaller annual changes in PaCO2 throughout long-term NIV had appreciably higher continuation rates of NIV.
Whereas general clinical status and ABGs are unstable in subjects before NIV, clinical status and gas exchange noticeably stabilize after the introduction of long-term NIV.10,12,13,19,20 Therefore, when identifying predictive risk factors, parameters observed after NIV initiation appear more valuable and reliable than those observed before the commencement of NIV.18,20,22 We previously showed that PaCO2 measured a few months after the start of long-term NIV is a possible prognostic factor in subjects with both restrictive thoracic disease18 and COPD.20 In the present study, we show that the annual change in PaCO2 may be the most important prognostic factor in subjects who receive NIV for > 2 y.
The decreased PaCO2 observed during long-term NIV in one third of our subjects was an unexpected finding. We had supposed that PaCO2 might increase even in subjects receiving long-term NIV, because their respiratory muscle capacity gradually reduces with aging. Amazingly, PaCO2 decreased gradually for > 8 y in 10 subjects. Two of these subjects changed from a ventilator using a preset volume to one with a preset pressure. The other ventilator settings were never changed in these 10 subjects. In 12 subjects without pulmonary lesions, 9 showed decreased PaCO2 during long-term NIV. Therefore, in those subjects without pulmonary lesions, PaCO2 may have a tendency to decrease during long-term NIV.
Nonetheless, PaCO2 increased in two thirds of our subjects, indicating that the adaptation mechanism that permits chronic hypercapnia may have occurred. In general, adaptations develop in difficult situations. Therefore, we hypothesized that the subjects with greater annual changes in PaCO2 were more likely to discontinue NIV than those subjects with a more stable PaCO2.
Hypercapnia is known to be a poor prognostic indicator.9 Windisch et al7 proposed that nighttime NIV, with ventilator settings targeted at maximally decreasing PaCO2, could decrease daytime PaCO2 sufficiently and provide further clinical benefits including a prolonged life.2 These reports support the common understanding regarding the advantageous effect of nighttime, high intensity NIV in reducing nighttime, and therefore daytime, PaCO2.23–25 The present study, however, showed that there was no significant difference in PaCO2 3–6 months after the start of long-term NIV among the 3 groups. Multivariate analysis indicated that, to predict prognosis, the annual change in PaCO2 during long-term NIV may be a more useful parameter than PaCO2 measured a few months after the initiation of long-term NIV. Therefore, we hypothesize that, to improve prognosis, PaCO2 should not only be reduced as low as possible within a few months of NIV initiation, it should also be stabilized throughout long-term NIV.
During routine clinical care, effort is seldom made to reduce PaCO2, even when PaCO2 increases to considerably high levels during long-term NIV.16,18,20 Although it remains unclear whether reducing PaCO2 is effective, we should try to stabilize PaCO2 throughout long-term NIV by changing the ventilator type, adjusting ventilator settings, increasing the daily time usage of NIV, changing interfaces, and adding nutritional management and pulmonary rehabilitation.
Conclusions
We found that subjects with smaller annual changes in PaCO2 throughout long-term NIV had significantly higher continuation rates of NIV. Thus, stabilizing PaCO2 throughout long-term NIV may be important for NIV continuance rates. Efforts to reduce PaCO2 should be made if PaCO2 increases at a greater rate during long-term NIV.
Footnotes
- Correspondence: Tomomasa Tsuboi MD PhD, Department of Respiratory Medicine. National Hospital Organization Minami-Kyoto Hospital, 11 Naka-Ashihara, Joyo City, Kyoto 610-0113, Japan. E-mail: tomomasa{at}kuhp.kyoto-u.ac.jp.
The authors have disclosed no potential conflicts of interest.
- Copyright © 2014 by Daedalus Enterprises