Abstract
BACKGROUND: Volume-targeted noninvasive ventilation (VT-NIV), a hybrid mode that delivers a preset target tidal volume (VT) through the automated adjustment of pressure support, could guarantee a relatively constant target VT over pressure-limited noninvasive ventilation (PL-NIV) with fixed-level pressure support. Whether VT-NIV is more effective in improving ventilatory status in subjects with acute hypercapnic respiratory failure (AHRF) remains unclear. Our aim was to verify whether, in comparison with PL-NIV, VT-NIV would be more effective in correcting hypercapnia, hence reducing the need for intubation and improving survival in subjects with AHRF.
METHODS: We performed a prospective randomized controlled trial in the general respiratory wards of 8 university-affiliated hospitals in China over a 12-month period. Subjects with AHRF, defined as arterial pH <7.35 and ≥7.25 and PaCO2 >45 mm Hg, were randomly assigned to undergo PL-NIV or VT-NIV. The primary end point was the decrement of PaCO2 from baseline to 6 h after randomization. Secondary end points included the decrement of PaCO2 from baseline to 2 h after randomization as well as outcomes of subjects (eg, need for intubation, in-hospital mortality).
RESULTS: A total of 58 subjects were assigned to PL-NIV (29 subjects) or VT-NIV (29 subjects) and included in the analyses. The decrement of PaCO2 from baseline to 6 h after randomization was not statistically different between the PL-NIV group and the VT-NIV group (9.3 ± 12.6 mm Hg vs 11.7 ± 12.9 mm Hg, P = .48). There were no differences between the PL-NIV group and the VT-NIV group in the decrement of PaCO2 from baseline to 2 h after randomization (6.4 ± 12.7 mm Hg vs 5.0 ± 15.8 mm Hg, P = .71) as well as in the need for intubation (17.2% vs 10.3%, P = .70), and in-hospital mortality (10.3% vs 6.9%, P > .99).
CONCLUSIONS: Regardless of whether a VT- or PL-NIV strategy is employed, it is possible to provide similar support to subjects with AHRF. (ClinicalTrials.gov registration NCT02538263.)
- acute hypercapnic respiratory failure
- noninvasive ventilation
- volume-targeted
- pressure-limited
- target tidal volume
- hypercapnia
Introduction
Noninvasive ventilation (NIV) is increasingly being used in the care of subjects suffering acute respiratory failure.1–5 Several lines of evidence strongly support its use in subjects with acute respiratory failure due to COPD exacerbations,6–12 acute cardiogenic pulmonary edema,13–16 and immunocompromised states17,18 as well as in facilitating early liberation from invasive ventilation.19–21 However, pressure-limited NIV (PL-NIV) with fixed-level pressure support, the most commonly used mode in clinical studies and practice,22,23 cannot ensure the delivered tidal volume (VT) to a constant target value, because the VT varies depending on dynamic changes in inspiratory effort, airway resistance, and chest-wall and lung compliance.24,25 By contrast, volume-targeted NIV (VT-NIV), a hybrid mode in which the ventilator measures or estimates delivered VT through a built-in pneumotachograph and automatically adjusts pressure support within a preset range to provide a VT as close as possible to a target VT set by the physician, is able to guarantee a target VT that is relatively constant compared with PL-NIV with fixed-level pressure support.26 Conceivably, VT-NIV may be more beneficial in improving ventilatory status than PL-NIV, particularly in subjects with acute hypercapnic respiratory failure (AHRF), for whom it is of critical importance to efficiently improve ventilatory status.
The majority of previous studies on VT-NIV have been performed mainly in subjects with obesity hypoventilation syndrome26–28 and chronic respiratory failure.29–31 In subjects with AHRF, there has been only one study performed to date; this study showed that VT-NIV was more effective in correcting PCO2 levels and facilitating the recovery of consciousness than PL-NIV.32 However, as acknowledged by its authors, this study was a case-control study, without randomization and with a small sample size. Therefore, whether VT-NIV in such subjects is more effective in improving ventilatory status than PL-NIV remains to be assessed.
We postulated that the use of VT-NIV would be more effective in correcting hypercapnia than PL-NIV, due to its advantage in providing a relatively constant target VT, hence reducing the need for intubation and improving survival in subjects with AHRF. Accordingly, we conducted a prospective randomized controlled trial to assess the efficacy of VT-NIV in comparison with PL-NIV.
QUICK LOOK
Current knowledge
NIV is typically delivered using a pressure-limited NIV mode (PL-NIV) with a fixed pressure support, and the delivered tidal volume (VT) varies depending on inspiratory effort and respiratory mechanics. Volume-targeted noninvasive ventilation (VT-NIV), a hybrid mode that delivers a target VT by automated adjustment of pressure support, may guarantee a relatively constant target VT over PL-NIV with fixed-level pressure support. However, VT-NIV has seldom been described in subjects with acute hypercapnic respiratory failure, in whom it is of critical importance to efficiently improve ventilatory status.
What this paper contributes to our knowledge
This study compared VT-NIV and PL-NIV in subjects with AHRF. No significant differences were found in the decrement of PaCO2, need for intubation, or in-hospital mortality between the 2 groups.
Methods
Subjects
We conducted a prospective randomized controlled trial in the general respiratory wards of 8 university-affiliated hospitals in China. All of the wards had staff experienced in delivering NIV. Systematic training for the diagnosis and treatment of AHRF as well as NIV application to AHRF was performed before recruiting subjects. The protocol was approved by the ethics committee at each hospital, and written informed consent was obtained from all subjects, their next of kin, or other surrogate decision makers, as appropriate.
Patients were considered eligible for the study if they were diagnosed with AHRF, defined as arterial pH <7.35 and ≥7.25 with PaCO2 >45 mm Hg. Patients with pH <7.25 were not included, because such individuals are conventionally transferred to the ICU due to the severity of their illness. Exclusion criteria were as follows: age <18 y; excessive amount of respiratory secretions or weak cough; upper airway obstruction; recent oral, facial, or cranial trauma or surgery; recent gastric or esophageal surgery; severe abdominal distention; active upper gastrointestinal bleeding; cardiac or respiratory arrest; PaO2/FIO2 <150 mm Hg; pneumothorax; severe ventricular arrhythmia or myocardial ischemia; severe hemodynamic instability despite fluid repletion and use of vasoactive agents; severe metabolic acidosis; lack of cooperation; and refusal to receive NIV.
Randomization
Randomization was accomplished by a computer-generated random number sequence, which was stratified according to the study center. The allocation sequence was concealed from the coordinating center by the use of numbered, opaque, and sealed envelopes. An independent telephone contact system was used for randomization. All of the centers participating in the study were immediately put in contact with the coordinating center to obtain a randomization number if a subject fulfilled the inclusion criteria. Within 1 h after the validation of inclusion criteria, subjects were randomly assigned to undergo either PL-NIV or VT-NIV in a 1:1 ratio.
Interventions
Both modes of ventilation were provided by the same noninvasive ventilator (FLEXO ST30, CURATIVE Medical, Suzhou, China). For PL-NIV, the inspiratory positive airway pressure (IPAP) was initially set at 10 cm H2O and was continuously adjusted by increments/decrements of 1–2 cm H2O, according to subjects' tolerance (up to 25 cm H2O), to obtain a VT of 8–10 mL/kg of predicted body weight and a breathing frequency of <25 breaths/min over NIV use. For VT-NIV, the target VT was set at 10 mL/kg of predicted body weight, with IPAP ranging from 10 up to 25 cm H2O. For both modes, expiratory positive airway pressure (EPAP) was set at 5–8 cm H2O, backup frequency was set at 10 breaths/min, rise time was set at 25–200 ms, and inspiratory time was set at a minimum of 0.5 s and a maximum of 2.0 s. An oronasal mask (Bestfit2 face mask, CURATIVE Medical) was used as a first choice for all subjects, but a nasal mask (Bestfit2 nasal mask, CURATIVE Medical) was optional if subjects did not tolerate the oronasal mask. A disposable single limb circuit was used, and a leak port was incorporated into the mask. Supplemental oxygen was supplied for all subjects through a port in the mask, with the flow adjusted to maintain oxygen saturation between 90 and 95%.
All subjects were encouraged to use NIV as much as possible during the first 6 h after randomization and at least 10 h/d. They were rigorously monitored at the bedside to ensure optimal NIV use. Disconnection from the ventilator was allowed for short periods, to clear secretions, drink water, or eat food, but was not scheduled.
In all subjects, the levels of IPAP and EPAP and the daily use of NIV were gradually decreased under conditions of clinical stability. Attempts to withdraw NIV were made if IPAP was 10 cm H2O for PL-NIV or ranged from 10 to 12 cm H2O for VT-NIV and if EPAP was 5 cm H2O and daily use was <5 h for both modes. Weaning was considered successful if subjects were able to sustain spontaneous breathing without signs of respiratory distress within 72 h after withdrawal, defined as the presence of ≥2 of the following: arterial pH <7.35; frequency >30 breaths/min; PaO2 <60 mm Hg or SpO2 <90% at FIO2 ≥0.4; retraction of the intercostal spaces, use of accessory respiratory muscles, or thoracic-abdominal paradoxical movement; and decreased consciousness, agitation, or diaphoresis.33
Endotracheal intubation was considered if either of the following criteria were met: arterial pH <7.25 with PaCO2 increased by >20% compared with the baseline or PaO2 <60 mm Hg despite maximum tolerated supplemental oxygen, and if at least one of the following criteria were met: clinical signs suggestive of severely decreased consciousness (eg, coma, delirium); excessive amount of respiratory secretions with weak cough; use of accessory respiratory muscles or thoracic-abdominal paradoxical movement; severe upper gastrointestinal bleeding with aspiration or vomiting; or severe hemodynamic instability despite fluid repletion and use of vasoactive agents. Once a subject fulfilled these criteria, the final decision for intubation was made by the attending physician with the consent of subjects' next of kin or other surrogate decision makers, as appropriate, which meant that subjects in both groups who met intubation criteria could be intubated or noninvasively ventilated.
The end of the protocol was defined as: presence of intubation criteria, with or without intubation; termination of NIV resulting from intolerance; death; hospital discharge; or 28 d after randomization.
Measurements and Outcomes
At enrollment, data with respect to the subjects' demographic characteristics, underlying disease, and history of NIV use were recorded. Vital signs were recorded at baseline (before randomization) and at 1, 2, 3, 4, 5, and 6 h after randomization. Arterial blood gases and supplemental oxygen flow were recorded at baseline (before randomization) and at 2 and 6 h after randomization.
The use of accessory muscles was assessed according to the following scale: 0 = no visible tonic or phasic use of neck muscles; 1 = neck muscles taut but with no respiratory modulation (ie, tonic activity); 2 = mild respiratory modulation in neck muscle contraction; 3 = moderate phasic activity (no supraclavicular or intercostal in-drawing); 4 = vigorous phasic activity with in-drawing; 5 = vigorous phasic activity with abdominal paradox.34 For the determination of PaO2/FIO2 while subjects were receiving NIV, FIO2 was calculated by using the following conversion factor: 21% + (3% × oxygen flow in L/min of supplemental oxygen). This conversion factor provides an approximation of percent oxygen delivered, is influenced by minute ventilation and breathing patterns, and may be inaccurate when air leakage occurs around the mask or through the mouth.35
All NIV parameters were continuously monitored by the built-in pneumotachograph and downloaded by ventilator software. Peak inspiratory pressure (PIP), EPAP, exhaled VT, exhaled minute volume (V̇E), and leak were recorded at 0, 2, and 6 h after randomization and were averaged over 5 min after the respective time points. The SD of VT and IPAP during the first 6 h for each subject, indicating individual variance, was produced by continuously monitored data. The daily use of NIV during the first 5 d after randomization was recorded using ventilator software.
The primary end point was the decrement of PaCO2 from baseline to 6 h after randomization. Secondary end points included the decrement of PaCO2 from baseline to 2 h after randomization as well as outcomes of subjects (need for intubation, actual intubation rate, duration of NIV, weaning success rate of NIV, intolerance rate of NIV, 28-d mortality, in-hospital mortality, length of hospital stay, and hospital costs). Subjects were followed up until death, hospital discharge, or 28 d after randomization for subjects discharged home before day 28.
Statistical Analysis
We expected that the decrement of PaCO2 from baseline to 6 h after randomization would be 10 mm Hg in the PL-NIV group, based on our previous clinical experience with subjects meeting eligibility criteria. Based on the assumption that the decrement of PaCO2 from baseline to 6 h after randomization would be 17.5 mm Hg in the VT-NIV group and the SD would be 10 mm Hg (equivalent to a coefficient of variation of 133%), a sample of 29 subjects in each group would be required to detect a difference of 7.5 mm Hg in the decrement of PaCO2 with 80% power and a 2-tailed α value of .05.
Continuous variables are presented as mean ± SD with normal distribution or as median (interquartile range) with non-normal distribution unless otherwise specified. Qualitative or categorical variables are presented as absolute numbers with percentages. The test of normality was performed using the Kolmogorov-Smirnov test, and the homogeneity of variances was performed using the Levene test. Continuous variables between the 2 groups were compared using the Student t test for variables with a normal distribution and the Mann-Whitney U test for variables with a non-normal distribution. Variables within the same group were compared using analysis of variance for repeated measures, and the Bonferroni adjustment for multiplicity of tests was applied for post hoc comparisons to ensure that the total error rate did not exceed 0.05. Qualitative or categorical variables were compared by a chi-square test with or without continuity correction. The Kaplan-Meier estimate-of-survival curve was used to determine the following within 28 d after randomization: the cumulative incidence of intubation, the cumulative probability of remaining on NIV, and the cumulative probability of survival. Curves between the 2 groups were compared using the log-rank test. All tests were 2-sided. Differences with P values of <.05 were considered statistically significant, except for those from multiple comparisons. Statistical analysis was performed with SPSS 19.0 (SPSS, Chicago, Illinois).
Results
Subjects
From January 2013 through December 2013, a total of 107 patients with AHRF were eligible for inclusion in the study, and 58 underwent randomization. A total of 29 subjects were assigned to the PL-NIV group and 29 to the VT-NIV group, and all of these subjects were included in the analysis (Fig. 1).
Flow chart. NIV = noninvasive ventilation.
The demographic and baseline characteristics of the subjects at entry into the study were similar in both groups (Table 1). There was no statistically significant difference in baseline PaCO2 between the 2 groups (P = .22; Table 1).
Subject Demographic and Baseline Characteristics
NIV Application
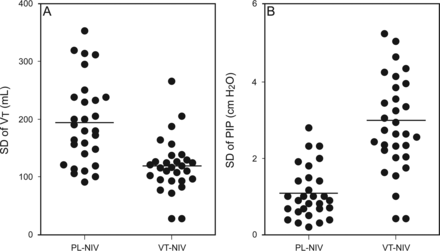
In subjects receiving VT-NIV, the preset target VT was 558.0 ± 62.0 mL. PIP, EPAP, exhaled VT, V̇E, and leak at 0, 2, and 6 h did not differ significantly between the 2 groups (P > .05; Table 2). During the first 6 h, the variance of VT was significantly lower in the VT-NIV group, as indicated by its lower individual SD (P < .001; Fig. 2). In contrast, the variance of PIP was significantly higher in the VT-NIV group, also as indicated by its higher individual SD (P < .001; Fig. 2). There was no difference in mean daily use of NIV during the first 5 d between the 2 groups (P > .05; Fig. 3).
Ventilator Parameters
SD of tidal volume (A) and peak inspiratory pressure (B) during the first 6 h after randomization. Points denote individual values and horizontal lines denote means. In both panels, P < .001 between the pressure-limited noninvasive ventilation group (PL-NIV) and volume-targeted noninvasive ventilation group (VT-NIV).
Daily use of noninvasive ventilation during the first 5 d after randomization. Values are given as means, and error bars denote SD. PL-NIV = pressure-limited noninvasive ventilation; VT-NIV = volume-targeted noninvasive ventilation.
Physiological Measurements
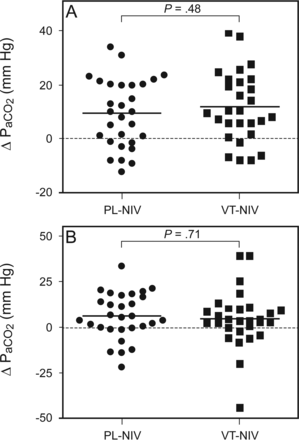
As shown in Figure 4A, the primary end point, the decrement of PaCO2 from baseline to 6 h after randomization, was not statistically different between the PL-NIV group and the VT-NIV group (9.3 ± 12.6 mm Hg vs 11.7 ± 12.9 mm Hg, P = .48). In addition, there was no difference in the decrement of PaCO2 from baseline to 2 h after randomization between the PL-NIV group and the VT-NIV group (6.4 ± 12.7 mm Hg vs 5.0 ± 15.8 mm Hg, P = .71) (Fig. 4B).
Decrement of PaCO2 from baseline to 6 h after randomization (A) and from baseline to 2 h after randomization (B). Points denote individual values, and solid horizontal lines denote means. PL-NIV = pressure-limited noninvasive ventilation; VT-NIV = volume-targeted noninvasive ventilation.
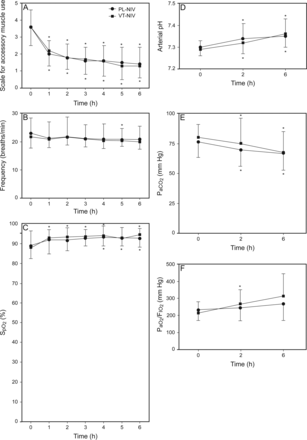
In both groups, there were significant and comparable within-group improvements over the first 6 h in scale for accessory muscle use, arterial pH, and PaCO2 (P < .05; Fig. 5, A, D, and E). Breathing frequency did not change significantly in the VT-NIV group, decreasing significantly only at 5 h in the PL-NIV group (Fig. 5B). SpO2 significantly improved over time in the VT-NIV group and began to improve only at 4 h in the PL-NIV group (Fig. 5C). PaO2/FIO2 significantly improved over time in the VT-NIV group and only tended to improve in the PL-NIV group (Fig. 5F). There were no between-group differences concerning PaCO2 or any of the other physiological parameters at any of the prespecified time points (P > .05; Fig. 5).
Scale for accessory muscle use (A), breathing frequency (B), SpO2 (C), arterial pH (D), PaCO2 (E), and PaO2/FIO2 (F) at baseline (0 h) and after randomization in subjects receiving pressure-limited noninvasive ventilation (PL-NIV) and volume-targeted noninvasive ventilation (VT-NIV). Values are given as mean ± SD. *, P < .05 compared with baseline within the same group.
Clinical Outcomes
The clinical outcomes of all of the subjects are shown in Table 3. There were no statistically significant differences in the need for intubation and the actual intubation rate between the 2 groups (P > .05). Likewise, the cumulative incidence of intubation within 28 d after randomization did not differ significantly between the 2 groups (P = .49 by log-rank test; Fig. 6A). There were no statistically significant differences in duration of NIV, intolerance rate of NIV, and NIV weaning success rate between the 2 groups (P > .05). The cumulative probability of remaining on NIV within 28 d after randomization did not differ significantly between the 2 groups (P = .40 by log-rank test; Fig. 6B). There were no statistically significant differences in the 28-d mortality and in-hospital mortality between the 2 groups, and the cumulative survival probability within 28 d after randomization did not differ significantly between the 2 groups (P = .62 by log-rank test; Fig. 6C). No significant differences were observed in the length of hospital stay and hospital costs between the 2 groups (P > .05).
Clinical Outcomes
Kaplan-Meier plot of the cumulative incidence of intubation (A), the cumulative probability of remaining on noninvasive ventilation (B), and the cumulative probability of survival (C) within 28 d after randomization. PL-NIV = pressure-limited noninvasive ventilation; VT-NIV = volume-targeted noninvasive ventilation. P values are by log-rank test.
Discussion
To the best of our knowledge, the present study is the first randomized controlled trial to perform VT-NIV in subjects with AHRF. The main finding of this study is that VT-NIV was as effective as PL-NIV in correcting hypercapnia, reducing the need for intubation, and improving survival in subjects with AHRF.
Our aim was to compare the effectiveness of VT-NIV and PL-NIV in subjects with AHRF. It is well known that PL-NIV in subjects with AHRF increases V̇E by increasing VT and allowing frequency to fall, thus improving PaCO2.6,36 However, during PL-NIV, fixed-level pressure support is provided by the ventilator, and the delivered VT cannot be guaranteed: It varies, depending on inspiratory effort, airway resistance, and chest-wall and lung compliance.24,25 Conceivably, PaCO2 may not be efficiently improved in some subjects, particularly in subjects with AHRF. By contrast, VT-NIV can guarantee a relatively constant VT, with a preset target VT and a pressure support adjusted automatically within a preset range to provide a VT as close as possible to the target VT.24,26 Therefore, in subjects with AHRF, it is possible that VT-NIV would lead to a more pronounced improvement in PaCO2 than PL-NIV.
Consistent with findings from previous studies6,10 and meta-analyses,11,12 PaCO2 was significantly improved after PL-NIV use. However, we found no more pronounced improvement in PaCO2 from baseline to 6 h and to 2 h after randomization in VT-NIV, and PaCO2 improved comparably 2–6 h after randomization in both groups. In contrast, Briones Claudett et al32 reported a case-control study that assessed the efficacy of VT-NIV in 22 subjects with AHRF, observing that 1–12 h after randomization, PaCO2 improved more significantly in VT-NIV than in PL-NIV. A possible explanation of the difference in results between the 2 studies is that PIP, VT, and V̇E were obviously higher in VT-NIV than in PL-NIV in the study of Briones Claudett et al,32 whereas these variables were not significantly different between the 2 groups in the present study.
In line with our study, Murphy et al28 demonstrated that in subjects with obesity hypoventilation syndrome, daytime PaCO2 improved similarly between the 2 modes, and Crisafulli et al30 also demonstrated that in subjects with stable hypercapnic COPD, PaCO2 improved comparably in both modes, with similar IPAP delivered in both modes. Nevertheless, Storre et al27 and Janssens et al26 reported that in subjects with obesity hypoventilation syndrome, mean transcutaneous PaCO2 improved significantly more in VT-NIV than in PL-NIV, whereas PIP and delivered IPAP in VT-NIV were higher than in PL-NIV. In addition, Storre et al31 found that although high-intensity NIV was performed to be compared with VT-NIV in subjects with chronic hypercapnic COPD, there remained no difference in the improvement of PaCO2, with similar PIP and VT being delivered. Therefore, PaCO2 was comparably improved in our study, possibly because in VT-NIV, the target VT that we preset in our protocol was 10 mL/kg, whereas in PL-NIV, the aim of the adjustment of IPAP by the clinician was also to obtain a VT of 8–10 mL/kg; consequently, a comparable pressure support level was delivered, and similar VT and V̇E were produced.
As expected, we found that in VT-NIV, the variance of VT was significantly lower, with a lower individual SD for each subject, suggesting that the delivered VT was more constant, and we also found that the actual VT was very close to the target VT in VT-NIV. However, on the basis of a similar pressure support level and delivered VT in both modes, VT-NIV did not lead to more pronounced improvement in PaCO2, at least over the first 6 h. Hence, keeping the delivered VT constant and close to the target value may not be responsible for the improvement in PaCO2; the pressure support level and VT value are more likely to play a key role in the improvement in PaCO2.
In accordance with the findings of previous studies6–9 and a meta-analysis,12 in which 6.7–25.6% of subjects needed intubation, we found that 17.2% of subjects in the PL-NIV group needed intubation. Furthermore, we found no difference in the need for intubation between the 2 modes. Two reasons may explain the similar efficacy of the 2 modes in the need for intubation. First, vital signs and arterial blood gases (especially pH and PaCO2) were comparably improved in both groups, suggesting comparable improvement of respiratory failure in both groups. Second, no difference was observed in the mean daily use of NIV during the first 5 d and the intolerance rate of NIV between the 2 groups, which indicated that subjects' comfort in the 2 modes was similar. It is of importance to note that NIV was terminated on the presence of NIV intolerance, regardless of the improvement in respiratory failure, and subjects were intubated when the hypercapnic respiratory failure progressively worsened after termination of NIV; thus, a similar NIV intolerance rate may lead to a similar need for intubation.
In line with the mortality of 9.3–10.2% shown in previous studies,6,7 in-hospital mortality was 10.3% in our study. Furthermore, we found no difference in in-hospital mortality between the 2 modes. Possible factors that may explain these findings are that there was no difference in baseline characteristics between the 2 groups, and the intubation rate was similar, suggesting that the improvement of the subject's clinical condition and respiratory failure may be similar, and the incidence of serious complications related to invasive ventilation (eg, ventilator-induced lung injury, ventilator-associated pneumonia) that are able to increase mortality may be no different between the 2 groups.37,38
Several limitations of this study should be taken into account. First, it is impossible to strictly blind the investigators and attending physicians in this type of open clinical trial, which may lead to possible bias. Despite the fact that we predefined criteria for all relevant interventions, clinical decisions, and outcome variables, such bias could not be entirely controlled. Second, the sample size is not large, which may lead to a low power of detection of significant between-group differences in the decrement of PaCO2; however, the planned sample size was achieved, and to the best of our knowledge, this is the largest clinical study to date on VT-NIV. Third, PaCO2 was comparably improved in both modes, possibly because the target VT was similar, so that actual pressure support level and VT were comparably produced. However, in cases where target VT in VT-NIV differs from PL-NIV, it is possible that VT-NIV would lead to a different improvement in PaCO2. Fourth, this study was performed in subjects with AHRF with arterial pH <7.35 and ≥7.25, suggesting that the results may not be generalizable to AHRF subjects with arterial pH <7.25 or ≥7.35. Finally, this study was undertaken at 8 hospital units with a great deal of experience with NIV use, suggesting that the results may not be generalizable to units without such experience.
Conclusions
Our study indicates that regardless of whether a VT- or PL-NIV strategy is employed, it is possible to provide similar support to patients with AHRF, and we suggest that VT-NIV may be considered as an alternative to PL-NIV in such patients.
Acknowledgments
We thank Dr Yichong Li for help in performing the statistical analysis.
Footnotes
- Correspondence: Zhixin Cao MD, Department of Respiratory and Critical Care Medicine, Beijing Engineering Research Center of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine, Beijing Chao-Yang Hospital, Capital Medical University, 5 Jingyuan Road, Shijingshan District, Beijing 100043, China. E-mail: caozhixinicu{at}126.com. Yingmin Ma MD, Department of Respiratory and Critical Care Medicine, Beijing Engineering Research Center of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine, Beijing Chao-Yang Hospital, Capital Medical University, 5 Jingyuan Road, Shijingshan District, Beijing 100043, China. E-mail: ma.yingmin{at}163.com.
See the Related Editorial on Page 1562
- Copyright © 2016 by Daedalus Enterprises