Abstract
BACKGROUND: The ventilatory ratio (VR) is a dead-space marker associated with mortality in mechanically ventilated adults with ARDS. The end-tidal alveolar dead space fraction (AVDSf) has been associated with mortality in children. However, AVDSf requires capnography measurements, whereas VR does not. We sought to examine the prognostic value of VR, in comparison to AVDSf, in children and young adults with acute hypoxemic respiratory failure.
METHODS: We conducted a retrospective study of prospectively collected data from 180 mechanically ventilated children and young adults with acute hypoxemic respiratory failure. VR was calculated as (minute ventilation ×  )/(age-adjusted predicted minute ventilation × 37.5). AVDSf was calculated as
)/(age-adjusted predicted minute ventilation × 37.5). AVDSf was calculated as  .
.
RESULTS: VR and AVDSf had a moderate correlation (rho 0.31, P < .001). VR was similar between survivors at 1.22 (interquartile range [IQR] 1.0–1.52) and nonsurvivors at 1.30 (IQR 0.96–1.95) (P = .2). AVDSf was lower in survivors at 0.12 (IQR 0.03–0.23) than nonsurvivors at 0.24 (IQR 0.13–0.33) (P < .001). In logistic regression and competing risk regression analyses, VR was not associated with mortality or rate of extubation at any given time (competing risk death; all P > .3). An AVDSf in the highest 2 quartiles, in comparison to the lowest quartile (AVDSf < 0.06), was associated with higher mortality after adjustment for oxygenation index and severity of illness (AVDSf ≥ 0.15–0.26: odds ratio 3.58, 95% CI 1.02–12.64, P = .047, and AVDSf ≥ 0.26: odds ratio 3.91 95% CI–1.03–14.83, P = .045). At any given time after intubation, a child with an AVDSf ≥ 0.26 was less likely to be extubated than a child with an AVDSf < 0.06, after adjustment for oxygenation index and severity of illness (AVDSf ≥ 0.26: subdistribution hazard ratio 0.55, 95% CI 0.33–0.94, P = .03).
CONCLUSIONS: VR should not be used for prognostic purposes in children and young adults. AVDSf added prognostic information to the severity of oxygenation defect and overall severity of illness in children and young adults, consistent with previous research.
Introduction
Physiologic dead space represents areas of the respiratory system that receive ventilation without perfusion and is composed of both anatomic dead space and alveolar dead space. Alveolar dead space can be elevated for numerous reasons in critically ill children, including alveolar overdistention and pulmonary hypoperfusion. Markers of physiologic and alveolar dead space have been consistently associated with mortality in mechanically ventilated children and adults with ARDS.1-5
Markers of dead space were considered for defining ARDS severity in both the Berlin definition of ARDS as well as the Pediatric Acute Lung Injury Consensus Conference pediatric ARDS definition.6,7 They were not included in either definition, partly due to the lack of routinely available established metrics to monitor dead space at the bedside. The most accepted estimate of physiologic dead space uses mean expired 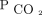 (
( ) in the Bohr-Enghoff equation, where the ratio of dead space to tidal volume (VD/VT) =
) in the Bohr-Enghoff equation, where the ratio of dead space to tidal volume (VD/VT) =  ).8
).8  is most easily measured with volumetric capnograpy; however, volumetric capnography is rarely used in routine clinical practice.
is most easily measured with volumetric capnograpy; however, volumetric capnography is rarely used in routine clinical practice.
We have previously reported that the end-tidal alveolar dead space fraction (AVDSf), calculated as  , is a useful marker of alveolar dead space in children.9 The AVDSf relies on routinely available time-based capnography data and has prognostic significance in mechanically ventilated children.1,2,9 For adults with ARDS, Sinha et al recently suggested the ventilatory ratio (VR) as a marker of dead space that can be calculated using routinely available clinical data without any measures of capnography.10-12 VR in adults is defined as:
, is a useful marker of alveolar dead space in children.9 The AVDSf relies on routinely available time-based capnography data and has prognostic significance in mechanically ventilated children.1,2,9 For adults with ARDS, Sinha et al recently suggested the ventilatory ratio (VR) as a marker of dead space that can be calculated using routinely available clinical data without any measures of capnography.10-12 VR in adults is defined as:

where  measured is measured minute ventilation,
measured is measured minute ventilation,  predicted is obtained by multiplying the predicted body weight by 100 mL/min/kg, and
predicted is obtained by multiplying the predicted body weight by 100 mL/min/kg, and 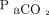 ideal is set at 37.5 mm Hg.
ideal is set at 37.5 mm Hg.
Sinha et al13 reported that, in adults with ARDS, VR is correlated with VD/VT and is independently associated with mortality. VR has not been studied in children with pediatric ARDS. The primary objective of this study was to investigate the association between a pediatric VR, adjusting for age-based differences in predicted 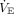 , and mortality in children and young adults with acute hypoxemic respiratory failure (all met pediatric ARDS oxygenation criteria, although the requirement for a new infiltrate on chest radiograph was not specifically confirmed). We compared the performance of VR against AVDSf in the study cohort.
, and mortality in children and young adults with acute hypoxemic respiratory failure (all met pediatric ARDS oxygenation criteria, although the requirement for a new infiltrate on chest radiograph was not specifically confirmed). We compared the performance of VR against AVDSf in the study cohort.
QUICK LOOK
Current Knowledge
Dead-space markers have been consistently associated with mortality in mechanically ventilated children and adults. The most well-accepted dead-space marker, calculated with the Bohr-Enghoff equation, requires measurement of mean expired 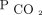 through monitoring devices that are generally not used in routine care. The ventilatory ratio is a dead-space marker that can be calculated from routinely available clinical data and is associated with mortality in mechanically ventilated adults with ARDS.
through monitoring devices that are generally not used in routine care. The ventilatory ratio is a dead-space marker that can be calculated from routinely available clinical data and is associated with mortality in mechanically ventilated adults with ARDS.
What This Paper Contributes to Our Knowledge
Ventilatory ratio was not associated with mortality and should not be used for prognostic purposes in mechanically ventilated children and young adults. The end-tidal alveolar dead space fraction, which uses routinely available time-based capnography measurements and is associated with mortality in mechanically ventilated children and young adults, is preferred for prognostic purposes.
Methods
This was a retrospective analysis of prospectively collected data from children and young adults admitted to the pediatric ICU at Children’s Hospital Los Angeles between January 2015 and December 2017. We identified all invasively mechanically ventilated patients < 21 y old who met pediatric ARDS oxygenation criteria (ie, oxygenation index [OI] > 4) or oxygen saturation index > 5) and had either arterial or capillary blood gases available for review.6 Subjects were included in this study if they had 2 OI or oxygen saturation index measurements, separated by 4 h, that both met oxygenation criteria. Patients were excluded if they had respiratory failure related to cardiac disease, an endotracheal tube leak > 20%, missing height data (ie, unable to estimate predicted body weight), or weight < 10 kg, and were on an AVEA ventilator (CareFusion, Yorba Linda, California) (ie, no automatic compensation for circuit compliance in volume calculations for the infant circuit). The study was approved by the Children’s Hospital Los Angeles Institutional Review Board (CCI-09-00126, CCI-09-00287).
Data Extraction and Procedures
We used 3 data sources for this study: continuous data from the bedside patient monitors (Philips Healthcare, Andover, Massachusetts), a copy of the electronic health record (Cerner, Kansas City, Missouri), and an administrative database, which updated with diagnostic and demographic information by clinicians providing direct patient care (Microsoft, Redmond, Washington). These databases are routinely monitored for accuracy through random sampling of records.
The timing of blood gases was determined by the clinical team. It is routine practice in our pediatric ICU to obtain blood gases during times of stability and not when ventilator changes or interventions have recently been performed (eg, endotracheal tube suctioning). Our pediatric ICU routinely monitors  with time-based capnography in all mechanically ventilated patients. Per unit policy, the
with time-based capnography in all mechanically ventilated patients. Per unit policy, the  module is calibrated using 2 points at setup and every 12 h thereafter. A pressure-targeted mode of ventilation is routine in our pediatric ICU. PEEP was selected at the discretion of the clinical team.
module is calibrated using 2 points at setup and every 12 h thereafter. A pressure-targeted mode of ventilation is routine in our pediatric ICU. PEEP was selected at the discretion of the clinical team.
Data from the first available blood gas were paired with ventilator and capnography data from the bedside monitor. Data were extracted in 30-s increments before and after the time of the blood gas. The mean value from the closest 4 data points to the blood gas for 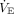 ,
,  , PEEP, peak inspiratory pressure,
, PEEP, peak inspiratory pressure,  ,
,  , and mean airway pressure were used in the analysis. We extracted data on demographics, diagnosis, 12-h Pediatric Risk of Mortality III (PRISM III) score, time to successful extubation (defined as remaining extubated for > 24 h), and mortality.14
, and mean airway pressure were used in the analysis. We extracted data on demographics, diagnosis, 12-h Pediatric Risk of Mortality III (PRISM III) score, time to successful extubation (defined as remaining extubated for > 24 h), and mortality.14
Variable Definition
Because the predicted 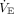 used in adults (100 mL/kg/min × predicted body weight) does not apply over the age range of children and young adults, a pediatric
used in adults (100 mL/kg/min × predicted body weight) does not apply over the age range of children and young adults, a pediatric  predicted was estimated.15,16 Pediatric predicted
predicted was estimated.15,16 Pediatric predicted 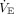 (mL/min) was estimated using the 50th-percentile age-based norm for breathing frequency, estimating 7 mL/kg for VT and multiplying by predicted body weight (see the supplementary materials at http://www.rcjournal.com).17 In secondary analyses, we used (1) the 10th-percentile age-based norm for breathing frequency and an estimated VT of 6 mL/kg; (2) the 90th-percentile age-based norm for breathing frequency and an estimated VT of 8 mL/kg; and (3) a previously published pediatric predicted VT (ie, 4.19 × height (cm) – 206.6) and the 50th-percentile age-based norm for breathing frequency to assess alternative methods of estimating pediatric
(mL/min) was estimated using the 50th-percentile age-based norm for breathing frequency, estimating 7 mL/kg for VT and multiplying by predicted body weight (see the supplementary materials at http://www.rcjournal.com).17 In secondary analyses, we used (1) the 10th-percentile age-based norm for breathing frequency and an estimated VT of 6 mL/kg; (2) the 90th-percentile age-based norm for breathing frequency and an estimated VT of 8 mL/kg; and (3) a previously published pediatric predicted VT (ie, 4.19 × height (cm) – 206.6) and the 50th-percentile age-based norm for breathing frequency to assess alternative methods of estimating pediatric  predicted for use in VR calculation.18 In 8 children, all under the age of 6 months, when the pediatric predicted VT equation yielded a VT < 10 mL, a value of 10 mL was imputed for the analysis.
predicted for use in VR calculation.18 In 8 children, all under the age of 6 months, when the pediatric predicted VT equation yielded a VT < 10 mL, a value of 10 mL was imputed for the analysis.
AVDSf is defined as 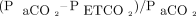 . Capillary
. Capillary 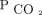 was used when arterial
was used when arterial 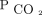 was not available. When a capillary blood gas was used, an oxygen saturation index was calculated (if
was not available. When a capillary blood gas was used, an oxygen saturation index was calculated (if  ≤ 97%) and converted to an OI equivalent as previously described.19 Predicted body weight was calculated using World Health Organization growth curves for infants and children < 2 y old and from Centers for Disease Control grids for those 2–20 y old. Δ Pressure was defined as the difference between peak inspiratory pressure and PEEP. Driving pressure (ie, the difference between plateau pressure and PEEP) was not available because it is not common in our clinical practice to obtain inspiratory holds to measure plateau pressure during pressure-regulated modes. Subjects whose cause of death was primarily related to neurologic disease were identified to determine if the performance of VR improved in the subgroup with non-neurologic death.
≤ 97%) and converted to an OI equivalent as previously described.19 Predicted body weight was calculated using World Health Organization growth curves for infants and children < 2 y old and from Centers for Disease Control grids for those 2–20 y old. Δ Pressure was defined as the difference between peak inspiratory pressure and PEEP. Driving pressure (ie, the difference between plateau pressure and PEEP) was not available because it is not common in our clinical practice to obtain inspiratory holds to measure plateau pressure during pressure-regulated modes. Subjects whose cause of death was primarily related to neurologic disease were identified to determine if the performance of VR improved in the subgroup with non-neurologic death.
Outcomes
Our primary outcome was pediatric ICU mortality. Rate of extubation at any given time after intubation was a secondary outcome.
Statistical Analysis
The correlation between AVDSf and VR was assessed with a Spearman correlation coefficient. We divided VR into quartiles and compared AVDSf across quartiles with a Kruskal-Wallis test. Pairwise comparisons were made with the Bonferroni correction.
Logistic regression was used to assess relationships between mortality and each dead-space marker. VR and AVDSf were modeled as categorical variables (quartiles) to meet model assumptions. Variables were considered for multivariable modeling if they had a univariable association (P < .2) with mortality or if there was substantial existing published evidence for inclusion (PRISM III, OI). Variables that changed the dead-space parameter estimate by > 15% were considered confounders. Interaction terms were considered. Area under the curve was calculated to describe model discrimination. Model fit was assessed with a Hosmer-Lemeshow test. A P value < .05 was considered significant. Analysis was performed using Stata 15 (StataCorp, College Station, Texas).
In secondary analyses, we used VR calculated using the 3 alternative methods of estimating a predicted pediatric 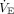 to determine if the association between VR and mortality was influenced by the assumptions we used in the primary analysis. We performed 3 sensitivity analyses: (1) limiting the analysis to non-neurologic deaths; (2) limiting the analysis to dead-space markers that were calculated with arterial
to determine if the association between VR and mortality was influenced by the assumptions we used in the primary analysis. We performed 3 sensitivity analyses: (1) limiting the analysis to non-neurologic deaths; (2) limiting the analysis to dead-space markers that were calculated with arterial  ; and (3) stratifying by the median age of 7 y (age categories: ≤ 7 y and > 7 y).20
; and (3) stratifying by the median age of 7 y (age categories: ≤ 7 y and > 7 y).20
Competing Risk Regression Analysis
For investigation of the secondary outcome of rate of extubation at any given time after intubation, we used a proportional hazards model for competing risks (ie, extubation was the primary outcome with the competing risk of death). This type of analysis allows for consideration of rate of extubation at any given time after intubation without noninformative censoring of subjects who die. Competing risk regression analyses, used to assess the duration of mechanical ventilation with consideration of subjects who die, are being used more frequently in critical care research due to analytic advantages over the use of ventilator-free days.21 We used the Fine and Gray method, which generates a subdistribution hazard ratio to estimate the rate of extubation at any given time after intubation while considering the competing risk of death.22 We tested the proportional hazard assumption using time-dependent covariates in the models.
Results
There were 200 children and young adults who met inclusion criteria; 20 of these were excluded, leaving 180 children and young adults in the study cohort. There were 97 males (53.9%), and the median age was 86.2 months (interquartile range [IQR] 15.5–160) (Table 1). Mortality was 26.1%, and duration of mechanical ventilation in survivors was 8.2 d (IQR 4.8–13.7). There were 15 (31.9%) deaths that were related primarily to neurologic disease. Median AVDSf was 0.15 (IQR 0.05–0.26), and median VR was 1.25 (IQR 1.0–1.6).
Baseline Demographics and Clinical Characteristics by Survival
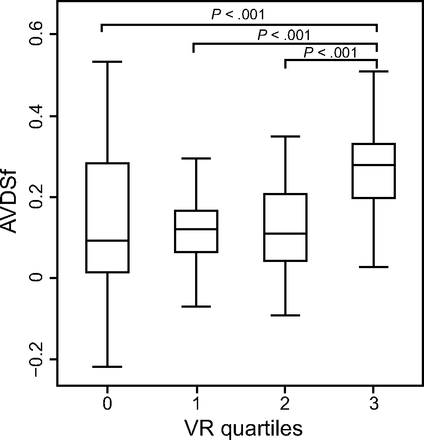
VR and AVDSf had a moderate correlation (rho 0.31, P < .001). When quartiles of VR were examined, AVDSf was significantly different across quartiles (P < .001) (Fig. 1). AVDSf was significantly lower in the first 3 VR quartiles than in the highest VR quartile (Q1: AVDSf 0.09, IQR 0.01–0.28; Q2: AVDSf 0.12, IQR 0.06–0.17; Q3: AVDSf 0.11, IQR 0.04–0.21; vs Q4: AVDSf 0.28, IQR 0.2–0.33; all P < .001).
End-tidal alveolar dead space fraction (AVDSf) by ventilatory ratio (VR) quartiles (Q1 < 1; Q2: ≥ 1–1.25; Q3 > 1.25–1.63; Q4 > 1.63). P values were determined with a Kruskal-Wallis test. The boxes represent the interquartile ranges with the median values represented by a bold line. Whiskers represent upper adjacent and lower adjacent values. Outliers are not shown.
Median VR was similar between survivors and nonsurvivors (1.22, IQR 1.0–1.52, vs 1.30, IQR 0.96–1.95, P = .20) (Table 1). VR was not associated with mortality in logistic regression modeling (Table 2). Nonsurvivors had a significantly higher median AVDSf than survivors (0.24, IQR 0.13–0.33, vs 0.12, IQR 0.03–0.23, P < .001). In multivariable logistic regression modeling, an AVDSf in the highest 2 quartiles, in comparison to the lowest quartile (AVDSf < 0.06), was associated with mortality after adjustment for OI and PRISM III: AVDSf ≥ 0.15–0.26: odds ratio 3.58 (95% CI 1.02–12.64), P = .047; and AVDSf ≥ 0.26: odds ratio 3.91 (95% CI 1.03–14.83), P = .045) (Table 2).
Logistic Regression Analyses for Association Between Dead Space Markers and Mortality
In the secondary analyses, VR was not associated with mortality when using any of the 3 alternative methods of calculating the predicted pediatric  (see the supplementary materials at http://www.rcjournal.com).
(see the supplementary materials at http://www.rcjournal.com).
Sensitivity Analyses
The first sensitivity analysis excluded death primarily related to neurologic causes (15 deaths excluded). The results were similar, and VR was not associated with mortality. The second sensitivity analysis restricted dead-space measurements (AVDSf or VR) to those that were calculated with an arterial 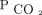 (eliminating 40 capillary
(eliminating 40 capillary  measurements). The results were similar, and VR was not associated with mortality. The third sensitivity analysis, stratifying by median age, revealed no association between VR and mortality in either age group (see the supplementary materials at http://www.rcjournal.com).
measurements). The results were similar, and VR was not associated with mortality. The third sensitivity analysis, stratifying by median age, revealed no association between VR and mortality in either age group (see the supplementary materials at http://www.rcjournal.com).
Rate of Extubation
In the secondary analyses, using a competing risk regression analysis, subjects in the lowest VR quartile had a similar rate of extubation at any given time after intubation to subjects in the higher 3 quartiles (all P > .30) (Table 3). At any given time after intubation, a child with an AVDSF ≥.26 was less likely to be extubated than a child with an AVDSF <0.06, after adjustment for OI and PRISM III (AVDSf ≥.26: subdistribution hazard ratio 0.55 (95% CI 0.33–0.94), P = .03).
Competing Risk Regression Analyses for Association Between Dead Space Markers and Rate of Extubation at Any Time After Intubation
Discussion
Our results indicate that VR is not associated with mortality or rate of extubation at any given time after intubation in children and young adults with acute hypoxemic respiratory failure. This is in contrast to the recent study by Sinha et al13 in adults with ARDS, who reported that VR was independently associated with mortality after adjusting for  /
/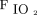 , PEEP, and severity of illness. We confirmed, consistent with our previous research, that AVDSf adds prognostic value to oxygenation markers and severity of illness in children and young adults with acute hypoxemic respiratory failure.1,2
, PEEP, and severity of illness. We confirmed, consistent with our previous research, that AVDSf adds prognostic value to oxygenation markers and severity of illness in children and young adults with acute hypoxemic respiratory failure.1,2
One of the reasons why VR does not perform as well as AVDSf for mortality discrimination in children and young adults with acute hypoxemic respiratory failure is due to the difficulty in estimating predicted  in children. In the adult VR equation, predicted
in children. In the adult VR equation, predicted  is based on 100 mL/kg/min and is normalized to predicted body weight. Children have differences in anatomic dead space and physiologic differences in oxygen consumption that change with age.15,16 These affect predicted
is based on 100 mL/kg/min and is normalized to predicted body weight. Children have differences in anatomic dead space and physiologic differences in oxygen consumption that change with age.15,16 These affect predicted  , making any estimated predicted
, making any estimated predicted 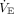 value invalid across the entire pediatric age spectrum. Neonates can have a predicted
value invalid across the entire pediatric age spectrum. Neonates can have a predicted 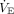 as high as 300 mL/kg/min.23,24 We attempted to address this by using a variety of methods to estimate age-adjusted predicted
as high as 300 mL/kg/min.23,24 We attempted to address this by using a variety of methods to estimate age-adjusted predicted  ; however, all of these calculations were based on assumptions and estimates, and none of them yielded a VR that performed well across pediatric age ranges.
; however, all of these calculations were based on assumptions and estimates, and none of them yielded a VR that performed well across pediatric age ranges.
There are additional reasons why VR may not perform well in children. Infants and younger children have smaller VT that may not be measured accurately by the ventilator, leading to inaccuracy of measured 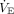 .25 The sensitivity analysis based on age groups, however, did not suggest that VR performed better in older children. In addition, the best method to predict body weight in children is controversial, with multiple proposed methods, all of which have limitations.26 It is often difficult to measure height accurately in recumbent children, particularly those with scoliosis or contractures, which are common comorbidities encountered in the pediatric critical care unit.27 Furthermore, while it is likely true in both obese adults and obese children that lung volume is best estimated from predicted body weight, children who have failure to thrive may in fact have smaller lung volumes than those estimated by predicted body weight.28 Additionally, the
.25 The sensitivity analysis based on age groups, however, did not suggest that VR performed better in older children. In addition, the best method to predict body weight in children is controversial, with multiple proposed methods, all of which have limitations.26 It is often difficult to measure height accurately in recumbent children, particularly those with scoliosis or contractures, which are common comorbidities encountered in the pediatric critical care unit.27 Furthermore, while it is likely true in both obese adults and obese children that lung volume is best estimated from predicted body weight, children who have failure to thrive may in fact have smaller lung volumes than those estimated by predicted body weight.28 Additionally, the 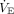 requirement can be elevated for other reasons than dead space, such as increased CO2 production from hypermetabolic states, which are not adequately captured in the VR equation. It is possible that differences in the underlying cause of ARDS in children versus adults, commonly indirect lung injury in adults and direct lung injury in children, may contribute to VR performing better in adult ARDS.
requirement can be elevated for other reasons than dead space, such as increased CO2 production from hypermetabolic states, which are not adequately captured in the VR equation. It is possible that differences in the underlying cause of ARDS in children versus adults, commonly indirect lung injury in adults and direct lung injury in children, may contribute to VR performing better in adult ARDS.
Both AVDSf and VR are affected to some degree by intrapulmonary shunt. The Enghoff modification of the Bohr equation for physiologic dead space makes the assumption that arterial  is equal to alveolar
is equal to alveolar  . This same assumption is true for VR and AVDSf calculation. When significant intrapulmonary shunt occurs, the arterial
. This same assumption is true for VR and AVDSf calculation. When significant intrapulmonary shunt occurs, the arterial 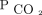 may be higher than alveolar
may be higher than alveolar 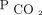 . However, this would artificially raise both VR and AVDSf and is therefore unlikely to be the reason behind the discrepant performance between VR and AVDSf in children and young adults.
. However, this would artificially raise both VR and AVDSf and is therefore unlikely to be the reason behind the discrepant performance between VR and AVDSf in children and young adults.
AVDSf remained independently associated with mortality after controlling for both OI and PRISM III. On the other hand, OI was no longer associated with mortality or rate of extubation at any given time after intubation after adjusting for AVDSf. From this analysis, AVDSf clearly adds prognostic information to oxygenation markers and PRISM III. Furthermore, in this analysis, AVDSf performed better than OI. This may be related to dead-space markers providing important information on adequacy of pulmonary perfusion and cardiac output that may not be captured by oxygenation metrics. Given that AVDSf is a simple measure that can be easily calculated in any intubated child monitored with time-based capnography and with an arterial or capillary blood gas, we believe it should be used as the primary marker for risk stratification in children because it balances ease of use with strong prognostic relevance.
AVDSf does require an invasive measure of 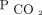 . Pediatric critical care practice has moved away from frequent arterial blood gases in all but the sickest patients.29 We used capillary
. Pediatric critical care practice has moved away from frequent arterial blood gases in all but the sickest patients.29 We used capillary 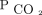 to calculate AVDSf for some subjects in this study and found consistent results with the subgroup limited to arterial
to calculate AVDSf for some subjects in this study and found consistent results with the subgroup limited to arterial 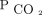 measurements. Therefore, we believe either an arterial blood gas or capillary blood gas can be used to estimate AVDSf with reasonable accuracy. We did not evaluate AVDSf for those with venous gases, which are increasingly used in pediatric critical care. Future investigations are needed to understand whether venous blood gases from a central line have any relevance in estimates of dead space in children. With the growing abundance of literature suggesting we are missing a key component of severity of lung disease when we focus only on oxygenation defect, alternative noninvasive methods for assessment of the ventilation defect must be considered. This may be through advanced analysis of the volumetric capnography curve as suggested by Fletcher et al30 and Tusman et al8,31 to obtain an estimate of alveolar
measurements. Therefore, we believe either an arterial blood gas or capillary blood gas can be used to estimate AVDSf with reasonable accuracy. We did not evaluate AVDSf for those with venous gases, which are increasingly used in pediatric critical care. Future investigations are needed to understand whether venous blood gases from a central line have any relevance in estimates of dead space in children. With the growing abundance of literature suggesting we are missing a key component of severity of lung disease when we focus only on oxygenation defect, alternative noninvasive methods for assessment of the ventilation defect must be considered. This may be through advanced analysis of the volumetric capnography curve as suggested by Fletcher et al30 and Tusman et al8,31 to obtain an estimate of alveolar  , or through consideration of noninvasive estimates of arterial
, or through consideration of noninvasive estimates of arterial 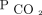 such as transcutaneous
such as transcutaneous  measurements.32
measurements.32
This study has some limitations. We did not have data from volumetric capnography to estimate physiologic dead space (VD/VT) using the Bohr-Enghoff equation. Although the correlation of VD/VT may have been stronger with VR, it would not have changed the association of VR with outcome. We also did not review chest imaging to verify that all subjects in the study met full pediatric ARDS criteria; therefore, it is possible that some subjects did not have pediatric ARDS. Finally, it is possible that VR performs better in a specific pediatric ARDS population.
Conclusions
VR should not be used for prognostic purposes in children and young adults. AVDSf is an easily measured dead space marker with prognostic significance (associated with mortality and risk of extubation at any given time after intubation) in children and young adults with acute hypoxemic respiratory failure.
Footnotes
- Correspondence: Anoopindar K Bhalla MD MSCI, 4650 Sunset Blvd MS #12, Los Angeles, CA 91214. E-mail: abhalla{at}chla.usc.edu
Dr Newth presented a version of this paper at the American Thoracic Society 2019 International Conference, held May 17–22, 2019, in Dallas, Texas.
Supplementary material related to this paper is available at http://www.rcjournal.com.
The authors have disclosed no conflicts of interest.
- Copyright © 2021 by Daedalus Enterprises